- No products in the cart.
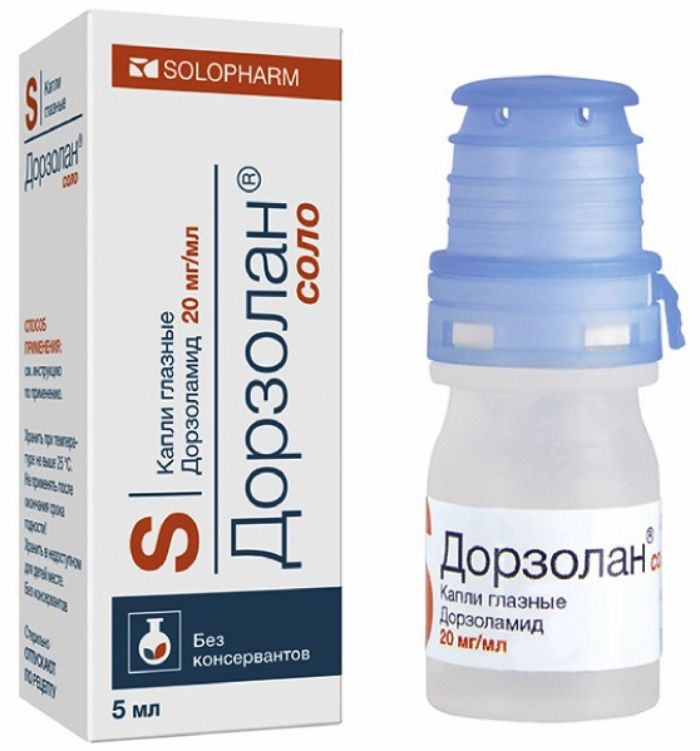
Dorzolan solo drops Ch. 20mg / 5ml ml vial 1 piece

Zinc sulfate-dia drops Ch. 10ml vial, cap.
$1.84

Katalin tab for prig.kapel Ch. + 0.75mg solvent 15ml vials.
$12.34
$9.08
Dorzolan solo drops Ch. 20mg / 5ml ml vial 1 piece
Description
Composition
Active substance:
1 ml contains: dorzolamide hydrochloride 22.26 mg, in terms of dorzolamide 20.0 mg.
Excipients:
Sodium citrate dihydrate 2.94 mg Sodium hyaluronate 1.80 mg, 23.0 mg mannitol, 1 M sodium hydroxide solution until pH 5,6, water for injection to 1.0 ml.
Description:
Clear, colorless or almost colorless, slightly viscous liquid.
Product form:
Eye drops of 20 mg / ml.
In a 0.4 ml tube dropper low density polyethylene or polypropylene.
5 or 10-tube drippers in a package of foil or film without it.
At 2, 4, 6, 12 or 18 packets out of the foil film with five tube-droppers, or 1, 2, 3, 6 or 9, a package of foil film 10, a tube-droppers or 10, 20, 30, 60 or 90 tube-droppers, together with instructions for use in a stack of cardboard.
5, 7, and 10 mL plastic vials, equipped with drip spout.
1 bottle together with instructions for use in a stack of cardboard.
Contraindications
Age younger than 1 week; hypersensitivity to the drug; chronic renal insufficiency; pregnancy; lactation.
Carefully.
The drug has not been studied in patients with severe hepatic impairment and therefore should be used in these patients with caution.
Dosage
20 mg / ml
Indications
The drug is given to adult patients with: -oftalmogipertenziey; Primordial open-angle glaucoma; -psevdoeksfoliativnoy glaucoma; -vtorichnoy glaucoma (anterior chamber angle without unit).
The drug is administered to children to treat glaucoma in children with 1 week as monotherapy or as an adjunct to the treatment of beta-blockers.
Interaction with other drugs
Special studies of drug interactions Dorzolan solo with other drugs was conducted. In clinical studies, the drug with dorzolamide eye drops was administered in combination with other drugs without adverse manifestations of drug-drug interaction, including eye drops of timolol and betaxolol, as well as systemic drugs: inhibitors of angiotensin-converting enzyme (ACE) inhibitors, calcium channel blockers, diuretics, nonsteroidal anti-inflammatory drugs (including aspirin), hormones (estrogen, insulin, thyroxine).
It does not exclude the possibility of mutual reinforcement of the systemic effects of carbonic anhydrase inhibitors for internal use and preparation Dorzolan solo at their simultaneous use. Combined treatment with drugs is systemic and local carbonic anhydrase inhibitors in clinical trials have not been investigated.
Dorzolan solo formulation is an inhibitor of carbonic anhydrase, and although applied locally, partially absorbed and may have systemic effects. In clinical studies with the use of drugs in the form of dorzolamide eye drops was not accompanied by breach of the acid-base balance. However, similar phenomena were observed in the application of carbonic anhydrase inhibitors, including as a result of drug-drug interactions with other drugs (as a manifestation of toxicity in patients receiving high doses of salicylates). Thus, in appointing a drug Dorzolan solo should not forget about the possibility of such drug-drug interactions.
The patient should inform the doctor about all the medicines which he uses or plans to use, including those sold over the counter.
We should pay particular attention to high doses of acetylsalicylic acid, as may increase toxicity.
Overdose
Symptoms.
Possible electrolyte disturbances, metabolic acidosis, and the occurrence of sleepiness, nausea, dizziness, headache, weakness, abnormal dreams, dysphagia.
Treatment.
Being symptomatic therapy aimed at maintaining vital functions. Should be monitored plasma concentration of electrolytes (especially potassium) values and blood pH.
pharmachologic effect
Pharmacological group:
Antiglaucoma agents – carbonic anhydrase inhibitor.
Pharmacodynamics:
Dorzolamide hydrochloride – an inhibitor of carbonic anhydrase II.
Inhibition of carbonic anhydrase (CA) in the ciliary body of the eyeball reduces the production of intraocular fluid, presumably due to deceleration of the synthesis of bicarbonate ions and their subsequent reduction to the excretion of sodium and fluids. The result is a reduction in the intraocular pressure (IOP).
Pharmacokinetics:
With prolonged use selectively accumulates in erythrocytes as a result of selective binding to the carbonic anhydrase II (CA-II), wherein the plasma concentration of free dorzolamide remains extremely low. Dorzolamide forms the only metabolite – N-dezetil dorzolamide-suppressant to a lesser extent than dorzolamide, SC-H enzyme and enzyme KA-I. Metabolite accumulates in erythrocytes contacting mainly with KA-I. Dorzolamide moderately bound to plasma protein (about 33%). Dorzolamide and its metabolite derived predominantly intact through the kidneys. After treatment dorzolamide eluted from erythrocytes unevenly, i.e., very intense in the beginning, which leads to rapid and substantial reduction in concentration, followed by a slow washout phase with a half-life of about 4 months.
Pregnancy and breast-feeding
Do not use this drug during pregnancy and lactation.
Conditions of supply of pharmacies
Prescription.
side effects
In clinical trials with the drug in the form of dorzolamide eye drops in 1108 patients administered as monotherapy or adjunctive therapy for treatment of beta blockers. Approximately 3% of patients drug was withdrawn due to adverse local reactions on the part of the eye, the most frequent of them were conjunctivitis and reaction from the eyelids.
Adverse events recorded during the study and during the post-registration period, classified according to frequency (very often (> 1/10), often (> 1/100
special instructions
In old age may increase sensitivity to dorzolamide (required dose reduction).
The effect on the ability of control of vehicles and mechanisms
In applying the drug is not recommended to drive and engage in potentially hazardous activities that require high concentration and psychomotor speed reactions.
Storage conditions
At temperatures above 25 ° C.
Keep out of the reach of children.
Dosing and Administration
In applying the drug Dorzolan solo usual dose is 1 drop in the affected eye (or both eyes) in the morning, afternoon and evening.
When replacing any antiglaucoma drug on Dorzolan solo should start treatment with Dorzolan solo the next day after the cancellation of the previous drug.
With the simultaneous application of the drug to other Dorzolan solo eye drops, they must be instilled at intervals of not less than 10 minutes.
Working with a tube-dropper: 1. Detach one tube-dropper. 2. Open the tube-IV (making sure that the solution is at the bottom tyubik- dropper, rotating movements rotate and separate valve). 3. The required amount of drip in the eye of the drug.
The dosage contained in tube-dropper, sufficient for a single instillation into both eyes. After a single use tube-IV should be discarded, even if the content remains.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | Grotex Ltd. |









There are no reviews yet.