- No products in the cart.
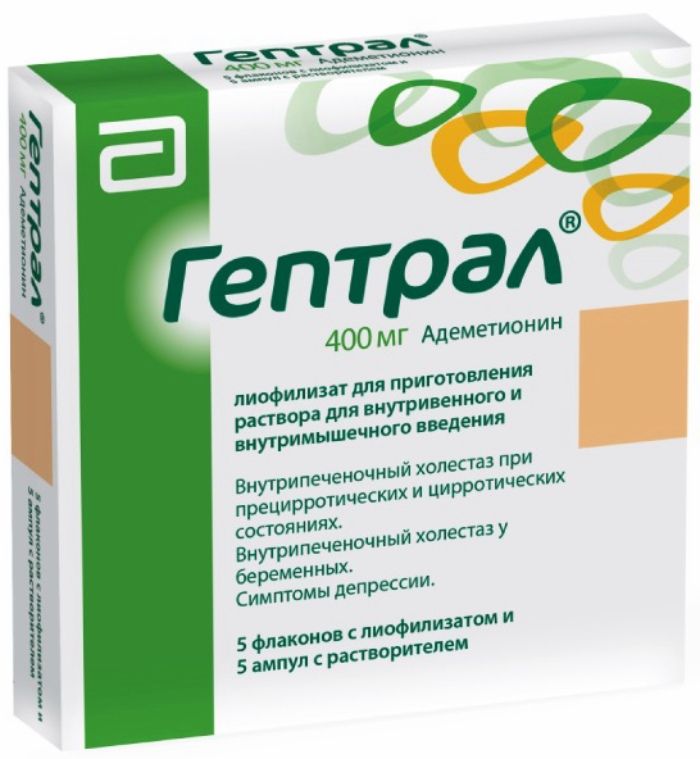
Geptral lyophilisates for solution for injection 400 mg vial with 5 pcs rastv.5ml Amp.
$38.90
Geptral lyophilisates for solution for injection 400 mg vial with 5 pcs rastv.5ml Amp.
SKU: 0180380375 Categories: Digestive tract, Hepatoprotectors, Medicaments Tags: Abbott, ademetionine
Description
Composition
Active substance:
1 vial of lyophilisate contains: 1,4-ademetionine butandisulfonat 760 mg (equivalent to 400 mg ion ademetionine).
Excipients:
Rastovritelem 1 ampoule contains: L-lysine 342.4 mg; 11.5 mg of sodium hydroxide; Water for Injection to 5 ml.
Description:
Lyophilisate: from almost white to white with a yellowish tint lyophilisate without extraneous inclusions.
Solvent: transparent liquid from colorless to pale yellow color with no foreign inclusions.
The reconstituted solution: clear solution colorless to yellow in color with no visible residue.
Product form:
760 mg of lyophilisate in a vial of colorless glass type I, chlorobutyl stopper capped with an aluminum cap with a plastic cap.
The solvent of 5 ml into ampoules of glass type I with a fracture point.
5 vials and ampoules 5 in a cardboard box, together with instructions for medical use.
5 vials and 5 vials in a plastic contour cell package covered with aluminum foil. 1 contour blister in a cardboard box with instructions for medical use.
Contraindications
Hypersensitivity to any component of the drug.
Age up to 18 years.
Carefully
Bipolar disorder (see. “Special Instructions” section).
Pregnancy (I term).
Lactation.
Dosage
80 mg / ml
Indications
Intrahepatic cholestasis and at pretsirroticheskih cirrhotic conditions that may occur in the following diseases: hepatic dystrophy -zhirovaya; -hronichesky hepatitis; -toksicheskie liver lesions of different etiology, including alcoholic, viral, drug (antibiotics, antineoplastic, and antitubercular antivirals, tricyclic -antidepressanty, oral contraceptives); -hronichesky acalculous cholecystitis; -holangit; -cirrhosis of the liver; -entsefalopatiya, including associated with liver failure (alcohol and others.).
Intrahepatic cholestasis in pregnancy.
Symptoms of depression.
Interaction with other drugs
Known interactions with other drugs, was not observed.
There are reports of serotonin syndrome, the excess in patients taking ademetionine and clomipramine. It is believed that this interaction is possible and should be used with caution ademetionine together with selective serotonin reuptake inhibitors, tricyclic antidepressants (such as clomipramine), as well as herbs and preparations containing tryptophan.
Overdose
Clinical cases of overdose were reported.
pharmachologic effect
Pharmacological group:
Hepatoprotective agent.
Pharmacodynamics:
Refers to hepatic ademetionine group also has antidepressant activity. And holekineticheskoe has choleretic effect, has detoxifying, regenerating, antioxidant, antifibroziruyuschimi and neuroprotective properties.
Fills deficit S-adenosyl-L-methionine (ademetionine) and stimulate its production in the body, it is found in all environments of the body. The highest concentration ademetionine observed in the liver and brain. It plays a key role in the metabolic processes of the body, taking part in important biochemical reactions: transmethylation, transsulfuration, transamination. In the reactions of transmethylation ademetionine donates a methyl group to the synthesis of phospholipids of cell membranes, neurotransmitters, nucleic acids, proteins, hormones, and others. In the reactions transsulfatirovaniya ademetionine is a precursor of cysteine, taurine, glutathione (providing redox mechanism cellular detoxification), coenzyme acetylation (included in biochemical reactions tricarboxylic acid cycle and replenishes the energy potential of the cells). Increases of hepatic glutamine, cysteine and taurine in plasma; It reduces the amount methionine in serum, normalizing the metabolic reactions in the liver. After decarboxylation reactions involved in aminopropilirovaniya, as a precursor of polyamines – putrescine (stimulator of cell regeneration and proliferation of hepatocytes), spermidine and spermine, within the structure of the ribosome, which reduces the risk of fibrosis. It has choleretic action. Ademethionine normalizes the synthesis of endogenous phosphatidylcholine in hepatocytes, which increases the fluidity and membrane polarization. This improves the function associated with the membranes of hepatocytes bile acid transport systems and facilitates passage of bile acids in the biliary system. Effective with intrahepatic (intralobular and interlobular) embodiment cholestasis (impaired synthesis and bile flow). Ademethionine reduces the toxicity of bile acids in the hepatocyte, exercising their sulfation and conjugation. The conjugation with taurine increases the solubility of bile acids and removing them from the hepatocyte. The process of sulfation of bile acids contributes to the possibility of elimination by the kidneys, facilitates passage through the membrane of hepatocytes and excretion in bile. In addition, the sulfated bile acids themselves further protect the membranes of liver cells from the toxic action of non-sulfated bile acids (present in high concentrations in hepatocytes in intrahepatic cholestasis). Patients with diffuse liver diseases (cirrhosis, hepatitis) syndrome intrahepatic cholestasis ademetionine reduces the severity of itching and changes in biochemical parameters, including level of direct bilirubin, alkaline phosphatase, aminotransferases et al. choleretic and hepatoprotective effect persists for up to 3 months after stopping treatment. The efficiency with hepatopathies resulting from different hepatotoxic drugs. Purpose of patients with opioid addiction, accompanied by liver disease leads to regression of clinical manifestations of withdrawal, to improve the functional state of the liver and microsomal oxidation processes. The antidepressant activity manifested gradually, starting from the end of the first week of treatment and stabilized for 2 weeks of treatment. Effective with recurrent endogenous and neurotic depression resistant to amitriptyline. It has the ability to interrupt the relapse of depression. Purpose in osteoarthritis reduces the severity of pain, increased synthesis of proteoglycans and lead to the partial regeneration of cartilage tissue.
Pharmacokinetics:
The bioavailability when administered parenterally – 96% in the plasma concentration reaches maximum values after 45 minutes. It is metabolized in the liver. Communication with plasma proteins – small. It penetrates the blood-brain barrier. Ademetionine been a significant increase in the CSF concentrations.
The half-life time (T1 / 2) -. 1.5 h excreted by the kidneys.
Pregnancy and breast-feeding
The use of high doses of ademetionina in the III trimester of pregnancy did not cause any adverse effects. Use of the drug in pregnant Geptral® I trimester and in the breast-feeding period is possible only if the potential benefit to the mother outweighs the potential risk to the fetus or child.
Conditions of supply of pharmacies
Prescription.
side effects
Among the most frequent adverse events observed: nausea, abdominal pain and diarrhea. The following are general data about the side reactions that occurred during treatment ademetionine both tablets and injectable dosage form.
Immune system: laryngeal edema, allergic reactions.
Skin: injection site reaction (very rare skin necrosis), sweating, itching, rash, angioedema, skin reactions.
Infections and infestations: urinary tract infection.
From the nervous system: dizziness, headache, paresthesia, confusion, and insomnia.
Cardio-vascular system: hot flashes, phlebitis of superficial veins, cardiovascular disorders.
On the part of the digestive system: abdominal distension, abdominal pain, diarrhea, dry mouth, dyspepsia, esophagitis, flatulence, gastrointestinal disorder, gastrointestinal bleeding, nausea, vomiting, colic, liver, cirrhosis of the liver.
On the part of the musculoskeletal system: arthralgia, muscle cramps.
Other: fatigue, chills, reaction at the site of administration, flu-like symptoms, malaise, peripheral edema, fever.
special instructions
Given the invigorating effect of the drug is not recommended to take it at bedtime. Geptral® when administering the drug to patients with cirrhosis amid hyperasotemia systematic control of nitrogen levels in the blood. During long-term therapy is necessary to determine the content of urea and creatinine in serum.
Do not appoint ademetionine patients with bipolar disorder. There are reports of depression going into hypomania or mania in patients taking ademetionine.
Patients receiving Geptral® is recommended to assign B12 and folic acid deficiency because tsianokobalomina (vitamin B12) and folic acid can reduce the concentration ademetionine.
Children
Safety of the drug in children Geptral® poorly understood.
Effects on ability to drive and use machines
In some patients, while taking the drug Geptral® can be dizziness. It is not recommended to drive a car and operate machinery while taking the drug for as long as patients are confident that the therapy does not affect engage in this type of activity for power.
Storage conditions
In the dark place at a temperature of from 15 ° C to 25 ° C. Keep out of the reach of children.
Dosing and Administration
Intravenously and intramuscularly.
Lyophilisate be dissolved in a specially enclosed solvent just prior to administration. The residue of the drug must be disposed of.
The drug can not be mixed with alkali solutions and solutions containing calcium ions.
Geptral® formulation at intravenous application introduced very slowly.
intrahepatic cholestasis
From 400 mg / day to 800 mg / day (1-2 vial per day) for 2 weeks.
Depression 400 mg / day (one bottle per day) during 15-20 days.
If necessary, maintenance therapy is recommended to continue receiving Geptral® drug in tablet form in doses 800-1600 mg / day for 2-4 weeks.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | Abbott |










There are no reviews yet.