- No products in the cart.
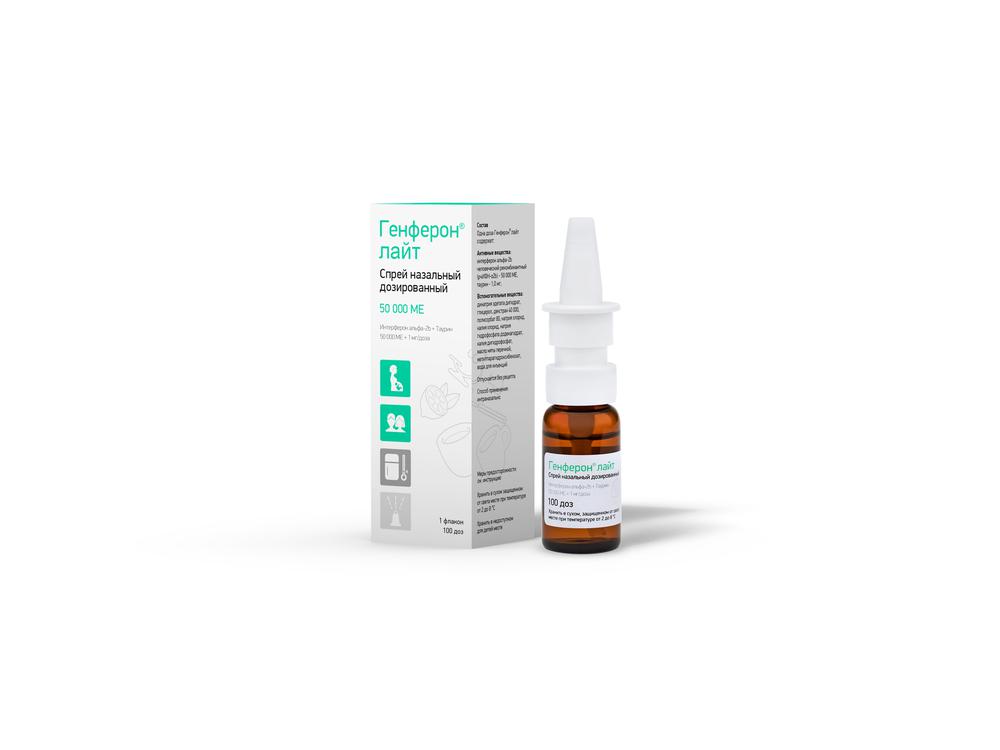
Genferon light spray Nazal. scrapper. 50000me + 1mg / dose 100doz

Oxoline 0.25% 10g tuba Nizhpharm
$1.76
Agri children (antigrippin gomeop.) Table 40 pcs / 20 pcs blister №1 №2 blister + 20 pcs /
$3.16
$24.60
Genferon light spray Nazal. scrapper. 50000me + 1mg / dose 100doz
SKU: 0778915447 Categories: Antiviral, immunocorrectors, Cold and flu, Medicaments Tags: interferon alfa-2b + taurine, OTISIFARM
Description
Composition
Active substance:
1 dose contains: interferon alpha-2b, recombinant human 50,000 IU, 1.0 mg taurine.
Excipients:
Disodium edetate dihydrate 0.02 mg, 7.0 mg glycerol, dextran 40 000 2.4 mg polysorbate 80 1.0 mg sodium chloride 0.8 mg, potassium chloride 0.02 mg, sodium hydrogen phosphate dodecahydrate 0.115 mg potassium dihydrogen phosphate 0.02 mg peppermint oil 0.01 mg methyl parahydroxybenzoate 0.02 mg water for injection sufficient quantity.
Description:
Transparent colorless or pale yellow liquid, without visible mechanical inclusions.
Product form:
100 doses in dark glass bottle, a sealed dispenser with a protective cap.
1 bottle with instructions for use in paper cartons.
Contraindications
Hypersensitivity to interferon alpha-2b, or other components of the preparation.
Children up to age 14 years.
Carefully.
It should be used with caution in patients with epistaxis.
Dosage
50,000 IU + 1 mg / dose
Indications
Prevention and treatment of influenza and acute respiratory viral infections in adults and children older than 14 years.
Interaction with other drugs
Not noted.
Overdose
No cases of overdose were reported GENFERON® LITE.
pharmachologic effect
Pharmacological group:
Immunomodulating agents, interferons.
Pharmacological properties:
The composition GENFERON® LITE preparation includes recombinant human interferon alpha-2b, produced by bacterial strain Escherichia coli, into which the genetically engineered gene introduced interferon alpha-2b human.
Interferon alpha-2b has antiviral, immunomodulatory, antiproliferative and antibacterial effects. The antiviral effect is mediated by the activation of a number of intracellular enzymes that inhibit the replication of viruses.
Immunomodulatory effects manifested, first of all, increased cell-mediated immune reactions, which increases the efficiency of the immune response against viruses, intracellular parasites and cells which have undergone a neoplastic transformation. This is achieved by activation of CD8 + killer T cells,
NK-cells (natural killer cells), enhancing the differentiation of B lymphocytes and their antibody production, activation of monocyte-macrophage system and phagocytosis as well as increasing the expression of molecules of the major histocompatibility complex type I, which increases the probability of detection of infected cells by cells of the immune system. Activation of leucocytes under influence of the interferon contained in all mucosal layers provides them with an active part in the elimination of lesions; In addition, due to the effect of interferon is achieved by restoring the production of secretory immunoglobulin A. The antibacterial effect is mediated by the immune system enhanced by the influence of interferon.
Taurine contributes to the normalization of metabolic processes and tissue regeneration, has a membrane and immunomodulatory effects. As a potent antioxidant, taurine interacts directly with the active oxygen species, an excessive accumulation of which promotes the development of pathological processes. Taurine helps to preserve the biological activity of interferon, enhancing the therapeutic effect of the drug.
Pharmacokinetics:
Intranasal application due to the high concentration in the infected site is achieved pronounced local antiviral and immunostimulating effect.
Systemic absorption is negligible – low bioavailability of drugs by nasal administration associated with the operation of a particular protein family of 25 proteins belonging to the nasal mucosa and controlling transportation of the molecular and cellular objects penetrating through the mucous.
At the same time, a certain amount of the drug reaches the systemic circulation, thereby achieving systemic immunomodulatory effect.
Pregnancy and breast-feeding
Allowed to use during the whole period of pregnancy.
Conditions of supply of pharmacies
Without recipe.
side effects
Side effects when using the drug GENFERON® LITE not observed.
Storage conditions
Be stored and transported in dry protected from light at a temperature of from 2 to 8 ° C.
Keep out of the reach of children.
Dosing and Administration
The drug used intranasally by aerosol administration of one dose (one dose = 1 doser short press).
At the first sign of disease GENFERON® LITE administered intranasally for 5 days, one dose (one press of the dispenser) into each nostril 3 times a day (single dose of about 50 000 IU of interferon alpha daily should not exceed 500 000 IU).
Upon contact with SARS patients and / or subcooled drug administered according to the scheme 2 times a day for 5-7 days. prevention courses are repeated, if necessary.
Instructions for use of the spray: 1. Remove the protective cap. 2. Before using for the first time, click on the dispenser several times until a fine spray. 3. Used keep the bottle upright. 4. Make the injection of the drug at a single press of the dispenser in each nostril in turn. 5. After use, close the protective cap dispenser.
To prevent the spread of infection is recommended individual use.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | OTISIFARM |










There are no reviews yet.