- No products in the cart.
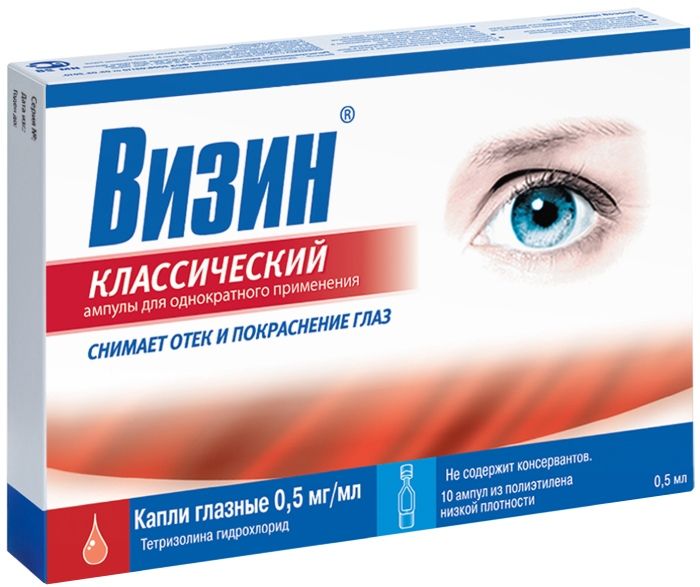
Visine classic drop Ch. 0.05% 0.5 mL ampoules 10 pieces

Taurine-solofarm drops Ch. 4% 0.4ml Tub-kap.p / ethyl. 20 pc
$1.69

Irifrin drops Ch. 2.5% 5ml vial
$13.74
$9.44
Visine classic drop Ch. 0.05% 0.5 mL ampoules 10 pieces
Description
Composition
Active substance:
1 ml of solution contains: tetryzoline hydrochloride – 0.50 mg ;.
Excipients:
Boric acid 12.30 mg / ml, sodium tetraborate (borax) 0.25 mg / mL sodium chloride 2.23 mg / ml, Water for injections to 1 mL.
Description:
The clear, colorless solution.
Product form:
Eye drops of 0.5 mg / ml.
0.5 ml of the product in clear vials for single use low density polyethylene. 5 vials are soldered together in a strip. 2 in the package strip of paper / PE / aluminum / film.
1 package together with instructions for use in a cardboard package.
Contraindications
Children under 2 years old.
Drug “Vizin® Classic” should not be used in patients with hypersensitivity to the drug, in patients with narrow-angle glaucoma and endothelial-epithelial corneal dystrophy.
CAREFULLY
In patients with severe cardiovascular disease (coronary heart disease, hypertension, arrhythmia, aneurysm), hyperthyroidism, pheochromocytoma, diabetes mellitus, and in patients receiving monoamine oxidase inhibitors or other agents that can raise blood pressure.
Dosage
0.5 mg / ml
Indications
Adults and children over 2 years assigned to relieve edema and conjunctival hyperemia (eye redness), resulting in allergy or caused by exposure to chemical and physical factors (smoke, dust, chlorinated water, light, cosmetics, contact lenses).
Children under 6 years of age shall be appointed under the supervision of a physician.
Interaction with other drugs
Preparation “Vizin® Classic” should not be used in conjunction with type tranylcypromine MAO inhibitors, tricyclic antidepressants, and drugs that increase blood pressure. Combination therapy with these groups of drugs can lead to increased vasoconstrictive effect and blood pressure.
Overdose
When used in accordance with the instructions the risk of overdose is minimal. However, in case of accidental contact with the drug in the gastrointestinal tract (ingestion), the following symptoms of overdose: mydriasis, nausea, cyanosis, fever, convulsions, tachycardia, arrhythmia, cardiac arrest, hypotension, pulmonary edema, inhibition of respiratory function, including apnea (cessation of breathing), depression of the central nervous system, including the development of drowsiness and coma.
The risk of developing symptoms of overdose due to systemic effects of the drug, is high in infants and young children, especially if swallowed.
A specific antidote is not known.
Treatment of overdoses when released into the gastrointestinal tract: activated charcoal, gastric lavage, inhalation of oxygen, antipyretic and anticonvulsants. To reduce arterial pressure is applied 5 mg phentolamine in saline slowly intravenously or 100 mg orally. Patients with low blood pressure pressor agents are contraindicated.
When any signs of overdose you should immediately contact a doctor !.
pharmachologic effect
Pharmacological group:
Alpha-agonists.
Pharmacodynamics:
Tetryzoline – sympathomimetic drug that stimulates alpha-adrenergic receptors of the sympathetic nervous system, but it does not have or has little effect on the beta-adrenergic receptors. As a sympathomimetic amine, tetryzoline has vasoconstrictor activity and reduces tissue edema.
The effect begins 60 seconds after instillation and lasts 4-8 hours.
Pharmacokinetics:
The local application is practically not absorbed. Detailed pharmacokinetic studies after topical application were not conducted eye drops.
Pregnancy and breast-feeding
Do not use the drug during pregnancy and breastfeeding, as well-controlled studies on the effect of tetryzoline hydrochloride on the fetus have not been conducted. Data on penetration of the drug through the placenta and breast milk are not available.
Conditions of supply of pharmacies
Without recipe.
side effects
Post-marketing data
Very common (> 1/10), common (> 1/100,
special instructions
“Vizin® Classic” should be used with caution in elderly patients, in patients with aneurysms, hypertension and / or coronary artery disease, and in patients with type I diabetes or hyperthyroidism.
safety studies of the drug in children and adolescents have not been conducted.
The preparation contains benzalkonium chloride which may cause eye irritation and change the color of soft contact lenses. Therefore, before the instillation of the drug is necessary to remove contact lenses and install them in 15 minutes.
Failure to comply with the instructions for use may develop reactive hyperemia of the conjunctiva and the mucous membranes of the nose (rhinitis medicamentosa).
If within 72 hours does not improve or irritation and redness persist or grow, it is necessary to cancel the use of the drug and seek medical advice. When the intense pain in the eyes, severe acute unilateral or red eyes, headaches, blurred vision, the appearance of spots before the eyes or double vision, immediately consult a doctor.
Long term use of the drug can enhance or cause flushing its reappearance.
If irritation or redness caused by diseases of the organ of vision (an infection, a foreign body or mechanical, chemical, thermal effects), then before applying the required medical consultation and determination of the need for further therapeutic measures.
Use of the drug can cause temporary expansion of the pupil.
Avoid prolonged use and drug overdoses.
Do not use the drug at a change in color or turbidity.
If the drug has come into disrepair or expiration date – do not pour it into the waste water and not be thrown into the street! Place the drug in the bag and place in the trash. These measures will help protect the environment!
EFFECTS ON THE ABILITY TO DRIVING AND USE OF TECHNOLOGY
In rare cases, after applying eye drops “Vizin® Classic” observed pupil expansion and there is blurred vision, which may affect the ability to drive and use machines.
Storage conditions
Store at a temperature not higher than 25 C.
Keep out of the reach of children.
Dosing and Administration
Locally. Adults and children over 2 years: 1 drop in the affected eye 2-3 times a day.
Use of the drug for more than 4 days should be taken only under medical supervision.
Instructions for use vials for single use
Each vial of the drug “Vizin® Classic” is designed for single use only. The drug should be used immediately after opening the ampoule. The amounts of the drug in a single vial is sufficient for a single administration in both eyes.
1. Break off one ampoule from the strip, and the remaining ampoules put back into the foil bag. 2. Open the vial by unscrewing the top, the unfilled part of the ampoule. 3. drip formulation in the conjunctival sac, gentle pressure on the filled part of the ampoule.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | JOHNSON |








There are no reviews yet.