- No products in the cart.
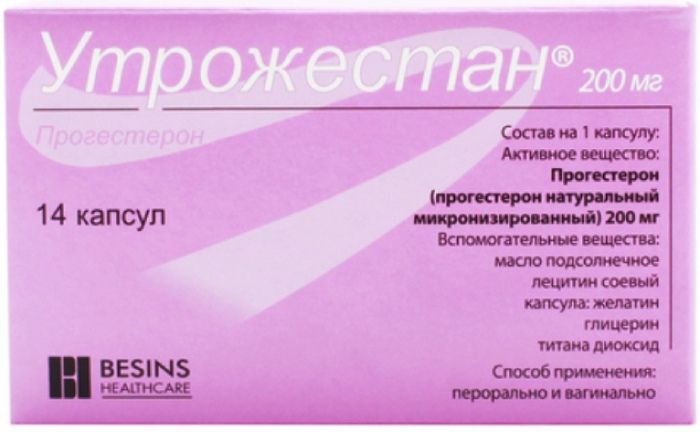
Utrozhestan caps. 200mg 14 pieces
$9.32
Utrozhestan caps. 200mg 14 pieces
Description
Composition
Active substance:
Micronized progesterone – 200 mg.
Excipients:
Sunflower oil – 149 mg / 298 mg, soy lecithin – 1 mg / 2 mg;
Capsule: gelatin – 76.88 mg / 153.76 mg Glycerin – 31.45 mg / 62.9 mg Titanium dioxide – 1.67 mg / 3.34 mg.
Description:
Oval soft gelatin capsule shiny yellowish oil containing whitish homogeneous suspension (without visible phase separation).
Product form:
7 capsules in blister PVC / aluminum foil and PVC / PVDC / aluminum foil. 2 blister with instructions for use in a cardboard bundle (14 capsules in consumer packaging).
Contraindications
Increased sensitivity to progesterone or any of the excipients of the formulation; deep vein thrombosis, thrombophlebitis; thromboembolic disorders (pulmonary embolism, myocardial infarction, stroke), or intracranial hemorrhage data availability conditions / diseases in history; bleeding from the vagina of unclear origin; incomplete abortion; porphyria; established or suspected malignancy of breast and genitals; severe liver disease (including cholestatic jaundice, hepatitis, Dubin-Johnson syndrome, Rotor, malignant liver tumors) now or in the anamnesis; Children up to age 18 years (effectiveness and safety have been established); during breastfeeding.
Carefully
Diseases of the cardiovascular system, hypertension, chronic renal failure, diabetes, asthma, epilepsy, migraine, depression, hyperlipoproteinemia, liver dysfunction and mild to moderate severity; photosensitivity. The drug should be used with caution in the II and III trimester of pregnancy.
Dosage
200 mg
Indications
Progesterondefitsitnye status of women.
For oral administration: threatened abortion or preventing habitual abortion due to insufficiency of progesterone; infertility due to insufficient luteal; premenstrual syndrome; menstrual disorders due to ovulation disorders or anovulation; fibrocystic breast disease; menopausal transition period; menopausal (substitution) hormone replacement therapy (MGT) in peri- and postmenopausal women (in combination with estrogen-containing preparations).
For intravaginal use:
MGT in the case of progesterone deficiency in dormant (absent), ovaries (egg donation); warning (prevention) preterm birth in women at risk (cervical shortening and / or presence of anamnestic data of preterm birth and / or premature rupture of membranes); luteal phase support in preparation for in vitro fertilization; support the luteal phase of spontaneous or induced menstrual cycle; premature menopause;
MGT (in combination with estrogen-containing preparations); infertility due to insufficient luteal; threatening abortion or prevention of habitual abortion due to progesterone deficiency.
Interaction with other drugs
For oral application
Progesterone enhances the action of diuretics, antihypertensive drugs, immunosuppressants, anticoagulants. Reduces the lactogenic effect of oxytocin. The simultaneous use of drugs, inducers of microsomal liver enzymes CYP3A4, such as barbiturates, antiepileptics (phenytoin, carbamazepine), rifampicin, phenylbutazone, spironolactone, griseofulvine, accompanied by an acceleration in the liver metabolism of progesterone. Simultaneous administration of progesterone with some antibiotics (penicillins, tetracyclines) may reduce its effectiveness due to violation of enterohepatic recirculation of sex hormones due to changes in the intestinal microflora. The severity of these interactions can be varied in different patients, and the prognosis of clinical effects of these interactions difficult. Ketoconazole may increase the bioavailability of progesterone. Progesterone can increase the concentration of ketoconazole and cyclosporin. Progesterone can reduce the effectiveness of bromocriptine. Progesterone may cause a decrease in glucose tolerance and thereby increase the need for insulin or other hypoglycemic agents in patients with diabetes. The bioavailability of progesterone can be reduced in smokers and patients with excessive alcohol consumption.
When intravaginal application
progesterone interaction with other drugs in intravaginal application was not evaluated. It should avoid the simultaneous use of other drugs used intravaginally, to avoid disturbing the release and absorption of progesterone.
Overdose
symptoms:
Somnolence, transient dizziness, euphoria, shortening of menstrual cycle, dysmenorrhea. In some patients the average therapeutic dose may be excessive due to existing or arising unstable endogenous progesterone secretion, a special sensitivity to the drug or too low concentration of estradiol.
Treatment: – in case of drowsiness or dizziness, is necessary to reduce the daily dose of the drug or an appointment before going to bed for 10 days of the menstrual cycle; – in the case of shortening of the menstrual cycle or “smearing” bleeding initiation of treatment is recommended to move to a later day cycle (e.g., 19 minutes instead of 17 th); – in perimenopausal and postmenopausal MGT need to make sure that the concentration of estradiol is optimal.
In overdose symptomatic treatment if necessary.
pharmachologic effect
Pharmacological group:
Progestogen.
Pharmacodynamics:
The active ingredient of the drug is progesterone Utrozhestan® identical to the natural corpus luteum hormone. By binding to the receptors on the surface of target organ cells, it enters the nucleus, where it activates DNA stimulates RNA synthesis. It translates between the endometrium from the proliferative phase induced follicular hormone estradiol in the secretory phase, and after fertilization – a condition necessary for the development of the fertilized ovum. Reduces the excitability and contractility of the muscles of the uterus and fallopian tubes. It promotes the formation of normal endometrium. It stimulates the development of breast end elements and induces lactation.
Stimulating proteinlipazu increases fat stores; increases glucose utilization; increasing the concentration of basal and stimulated insulin promotes the accumulation of glycogen in the liver; It increases the production of gonadotropic hormones of the pituitary; reduces azotemia, increases the excretion of kidney nitrogen.
Pharmacokinetics:
If ingestion
Suction
Micronized progesterone is well absorbed in the gastrointestinal tract (GIT). progesterone plasma concentration gradually increases during the first hour, the maximum plasma concentration (Cmax) observed after 1-3 hours after administration. The concentration of progesterone in blood plasma increases from 0.13 ng / ml to 4.25 ng / ml after 1 hour, up to 11.75 ng / mL at 2 hour and was 8.37 ng / ml after 3 hours 2 ng / ml over 6 hours, and 1.64 ng / mL at 8 hours after ingestion.
Metabolism
The major metabolites are determined in blood plasma, are 20-alpha-hydroxy-delta-4-alpha-pregnanolon and 5-alpha dihydroprogesterone.
breeding
Excreted by the kidneys in the form of metabolites to 95% of them are glyukuronkonyugirovannye metabolites, mostly 3-alpha, 5-beta-pregnanediol (pregnandion). These metabolites, which are determined in plasma and urine are similar to the substances formed during physiological secretion of the corpus luteum.
For vaginal administration
Absorption and distribution
Absorption is rapid, high concentration of progesterone in the blood plasma is observed 1 hour after administration. Cmax progesterone plasma levels achieved in 2-6 hours after administration. When administered 100 mg 2 times a day average plasma concentration is maintained at 9.7 ng / ml for 24 hours. When administered at doses of 200 mg / day, progesterone concentration corresponds to I trimester of pregnancy. Relationship to plasma proteins – 90%. Progesterone accumulates in the uterus.
Metabolism
Predominantly metabolized to form 3-alpha, 5-beta-pregnanediol. The concentration of 5-beta-pregnanolona not increased in blood plasma.
breeding
Excreted by the kidneys as metabolites, the main part is 3-alpha, 5-beta-pregnanediol (pregnandion). This is confirmed by the constant increase in its concentration (Cmax 142 ng / ml after 6 hours).
Pregnancy and breast-feeding
The drug should be used with caution in the II and III trimester of pregnancy due to the risk of cholestasis.
Progesterone passes into breast milk, therefore the use of the drug is contraindicated during breast-feeding.
Conditions of supply of pharmacies
On prescription.
side effects
The following adverse events reported by the oral route of administration, the frequency of occurrence distributed according to the following gradation: often> 1/100, 1/1000, 1/10000,
Violations by the genitals and breast often: menstrual disorders, amenorrhea, acyclic bleeding; rare: mammalgia
Violations by the psyche is very rare: depression
Disorders of the nervous system common: headache; rare: drowsiness, transient dizziness
Violations by the gastrointestinal tract common: bloating; uncommon: vomiting, diarrhea, constipation; rare: nausea
Disorders of the liver and biliary tract: infrequently: cholestatic jaundice
Violations by the immune system is very rare: urticaria
Violations of the skin and subcutaneous tissue disorders common: pruritus, acne;
Very rare: chloasma
Drowsiness, dizziness, possible transient is usually 1-3 hours after oral ingestion. These unwanted reactions may be reduced by lowering the dose of the drug application before bedtime or transition to the vaginal route of administration.
These undesirable reactions are usually the first signs of overdose.
Drowsiness and / or transient dizziness are observed, in particular, in the case of concomitant hypoestrogenism. dose reduction or a higher recovery estrogenizatsii immediately eliminates these effects, without reducing the therapeutic effect of progesterone.
If treatment begins too early (in the first half of the menstrual cycle, especially before the 15th day), there may be shortening of the menstrual cycle or acyclic bleeding.
Recorded changes in the menstrual cycle, amenorrhea or acyclic bleeding are common to all of progestogens.
special instructions
Utrozhestan® The drug should not be used for contraception. The drug should not be taken with food, as food intake increases the bioavailability of progesterone. Utrozhestan® drug should be used with caution in patients with diseases and conditions that may be exacerbated with fluid retention (hypertension, cardiovascular disease, chronic renal failure, epilepsy, migraine, bronchial asthma); in patients with diabetes mellitus, impaired liver function with mild to moderate severity with photosensitivity. It is necessary to monitor the patient with a history of depression, and in the case of severe depression need to remove the drug. The composition includes a preparation Utrozhestan® soya lecithin, which may cause hypersensitivity reactions (urticaria and anaphylactic shock). Patients with concomitant cardiovascular diseases or a history of their presence must also periodically observed physician. Use of the drug Utrozhestan® after I trimester of pregnancy can cause cholestasis development. With long-term treatment of progesterone necessary medical examinations on a regular basis (including liver function tests); Treatment must be canceled in the event of deviations from the normal values of liver or cholestatic jaundice functional tests. In the application of progesterone may be reduced glucose tolerance and increased demand for insulin and other hypoglycemic agents in patients with diabetes. In the case of amenorrhea during treatment, it is necessary to exclude the presence of pregnancy. If treatment begins too early, at the beginning of the menstrual cycle, especially before the 15th day of the cycle, there may be shortening the cycle and / or acyclic bleeding. In the case of acyclic bleeding should not use the drug to determine their causes, including histological examination of the endometrium. With a history of chloasma or the propensity to develop its patients are advised to avoid UV exposure. More than 50% of cases of spontaneous abortion in early pregnancy due to genetic disorders. In addition, the cause of miscarriages in early pregnancy may be infectious processes and mechanical damage. Use of the drug Utrozhestan® in these cases can only lead to rejection and delayed evacuation of non-viable ovum. Use of the drug Utrozhestan® to prevent threatened abortion is justified only in cases of progesterone deficiency. When conducting MGT estrogen during the perimenopause use is recommended Utrozhestan® drug for at least 12 days of the menstrual cycle. In continuous mode MGT postmenopausal recommended use of the drug on the first day of estrogen. In conducting MGT increases the risk of venous thromboembolism (deep vein thrombosis or pulmonary embolism), the risk of ischemic stroke, coronary heart disease. Because of the risk of thromboembolic complications should stop using the product in the event of: visual disturbances (such as loss of vision, proptosis, diplopia, vascular lesions of the retina), migraine, venous thromboembolism or thrombotic complications, regardless of their location. In the presence of thrombophlebitis in history, the patient should be monitored carefully. In applying the drug with estrogen-containing medications Utrozhestan® necessary to refer to the instructions for using the relative risk of venous thromboembolism. Results of clinical studies Women Health Initiative Study (WHI) indicate a small increase in breast cancer risk when prolonged over 5 years, joint application estrogensoderjath preparations with synthetic progestins. It is unknown whether the increased risk of breast cancer in postmenopausal women available in during MGT estrogensoderzhaschie drugs in combination with progesterone. WHI study also found an increased risk of dementia at the beginning of the MGT over the age of 65 years. Before the start of MGT and regularly during it a woman should be screened for contraindications to its performance. In the presence of clinical indications should be conducted breast examination and pelvic examination. The use of progesterone may affect the results of certain laboratory tests, including liver function tests, thyroid; coagulation parameters; pregnanediol concentration.
The effect on the ability of control of vehicles and mechanisms
Upon oral administration of the drug should be careful when driving and occupation of other potentially hazardous activities that require high concentration and psychomotor speed reactions.
Storage conditions
Store at a temperature not higher than 25 ° C.
Keep out of the reach of children.
Dosing and Administration
orally
The drug is taken orally in the evening before bedtime with water.
In most cases, the failure Utrozhestan® progesterone daily dose is 200-300 mg drug, divided into two doses (200 mg in the evening before going to bed, and 100 mg in the morning, if necessary).
When the threatened abortion or for preventing habitual abortion due to insufficiency of progesterone: 200-600 mg a day every day I and II trimester of pregnancy. Further application of formulation may Utrozhestan® prescribed by a doctor based on an evaluation of clinical data of the pregnant woman.
When insufficient luteal phase (premenstrual syndrome, fibrocystic breast disease, dysmenorrhea, menopausal transition), the daily dose is 200 or 400 mg taken within 10 days (usually 17 to 26-day cycle).
When MGT perimenopausal in patients receiving estrogen Utrozhestan® used at 200 mg per day for 12 days.
When MGT postmenopausal continuous preparation mode is applied in a dose of 100-200 mg from day reception estrogensoderjath preparations. dose selection is carried out individually.
intravaginal
Capsules are injected deep into the vagina.
Warning (prevention) preterm birth in women at risk (cervical shortening and / or presence of anamnestic data of preterm birth and / or premature rupture of membranes): the usual dose is 200 mg at bedtime to 22 minutes on the 34th week pregnancy.
The complete absence of progesterone in women with non-functioning (absent) ovaries (egg donation) on the background of estrogen therapy on 100 mg per day on the 13th and 14th days of the cycle, followed by 100 mg 2 times a day from 15 to 25 th day of the cycle, from the 26th day in the case of determining pregnancy dose increases to 100 mg per day, every week, reaching a maximum of 600 mg per day, divided into 3 doses. Said dose may be applied for 60 days.
Support the luteal phase during cycles of in vitro fertilization: It is recommended to use from 200 to 600 mg per day, beginning on the day of injection of human chorionic gonadotropin for I and II trimester.
Поддержка лютеиновой фазы в спонтанном или индуцированном менструальном цикле при бесплодии, связанном с нарушением функции желтого тела: рекомендуется применять 200-300 мг в сутки, начиная с 17-ого дня цикла на протяжении 10 дней, в случае задержки менструации и диагностики беременности лечение должно быть продолжено.
В случаях угрожающего аборта или в целях предупреждения привычного аборта, возникающих на фоне недостаточности прогестерона: 200-400 мг в сутки в 2 приема ежедневно в I и II триместрах беременности.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | BEZEN |










There are no reviews yet.