- No products in the cart.
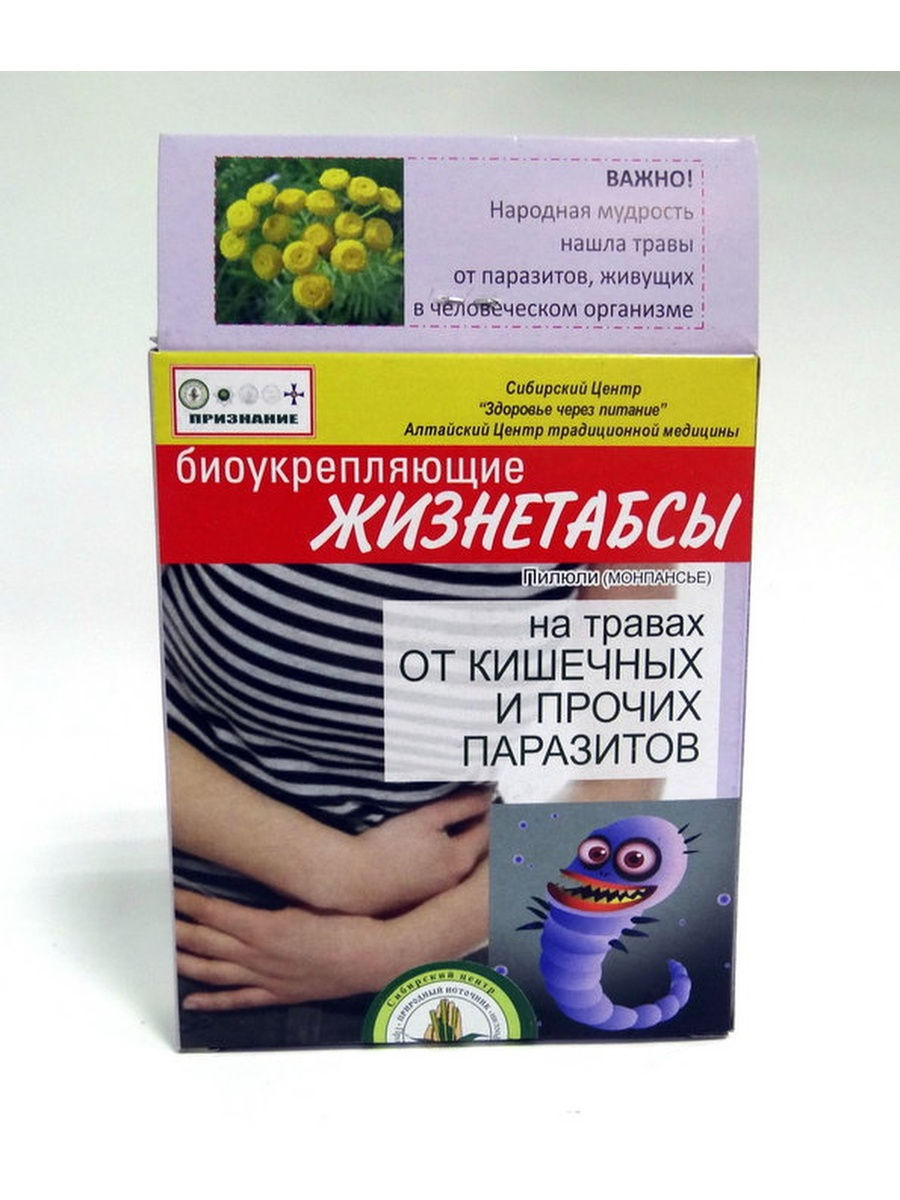
Tsefekon etc. Suppositories 50mg 10 pc

Nurofen for children oral suspension 100mg / 5ml 150ml vial with a syringe dosing orange
$4.17

Rinzasip powder for r-ra 5g 5 pcs black currants / Vitamin C.
$3.86
$0.83
Tsefekon etc. Suppositories 50mg 10 pc
Description
Composition
Active substance:
1 suppository contains paracetamol – 50 mg.
Excipients:
Suppository bases: solid fat (Witepsol, supposir) – to obtain a suppository weighing 1.25 g
Description:
Suppositories are white or white with yellowish or Valium a color shade, torpedo-shaped.
Product form:
Tsefekon® D rectal suppositories for children, 50 mg. 5 suppositories in blisters; Two blisters with instruction for medical use the drug is placed in a cardboard box.
Contraindications
Hypersensitivity, the neonatal period (up to 1 month).
Dosage
50 mg
Indications
Used in children from 3 months to 12 years as:
antipyretic acute respiratory diseases, influenza, childhood infections, post-vaccination reactions and other conditions involving fever; painkillers for pain syndrome mild to moderate intensity, including: headache, toothache, muscle pain, neuralgia, pain, trauma, and burns.
Children from 1 to 3 months is possible a single dose of the drug to reduce fever after vaccination, use of the drug in all indications can only be prescribed by a doctor.
Interaction with other drugs
Stimulants microsomal oxidation in the liver (phenytoin, ethanol, barbiturates, flumetsinol, rifampicin, phenylbutazone, tricyclic antidepressants), ethanol and hepatotoxic drugs increase the production of hydroxylated active metabolites, which leads to the possibility of severe intoxication, even with a small overdose. Inhibitors of microsomal oxidation (including cimetidine) decrease the risk of hepatotoxicity. When taken together with the nephrotoxic action of paracetamol salicylates increases. The combination with chloramphenicol, leads to an increase of the toxic properties of the latter. Potentiates the effect of indirect anticoagulants, reduces the effectiveness of uricosuric drugs.
Overdose
Symptoms: during the first 24 hours after administration – paleness of the skin, nausea, vomiting, anorexia, abdominal pain; impaired glucose metabolism, metabolic acidosis. Symptoms of liver function may occur through 12-48 hours after the overdose. In severe overdose – liver failure with progressive encephalopathy, coma, and death; acute renal failure with tubular necrosis (including the absence of severe liver disease); arrhythmia, pancreatitis. Hepatotoxic effect manifests itself in adults when receiving 10 g or more.
Treatment: administering donators SH-groups and precursors of glutathione synthesis – methionine through 8-9 hours after the overdose and N-acetylcysteine - after 12 hours. The need for further therapeutic activities (methionine further administration, intravenous administration of N-acetylcysteine) is determined depending on the blood concentration of paracetamol, as well as the time elapsed after administration.
pharmachologic effect
Pharmacological group:
Non-narcotic analgesic agent.
Pharmacodynamics:
Paracetamol has analgesic and antipyretic action. The drug blocks the cyclooxygenase in the central nervous system, acting on pain centers and thermoregulation. In inflamed tissues cellular peroxidase neutralize the effect of paracetamol on the cyclo-oxygenase, which explains the lack of significant anti-inflammatory effect.
The absence of a blocking effect on prostaglandin synthesis in peripheral tissues it causes no adverse effect on the water-salt exchange (sodium and water retention) and the mucous of the gastrointestinal tract.
Pharmacokinetics:
High absorption, is rapidly absorbed from the gastrointestinal tract. The period to reach maximum concentration – 30-60 minutes. It penetrates the blood-brain barrier. The value of Bioavailability in children and neonates are similar to those in adults. It is metabolized in the liver. The half-life – 2-3 hours. Within 24 hours, 85-95% paracetamol excreted by the kidneys in the form of glucuronides and sulfates unchanged – 3%. Significant age difference in the elimination rate of paracetamol and in the total amount of the drug that is excreted in the urine, no.
Conditions of supply of pharmacies
Without recipe.
side effects
Allergic reactions (including rash).
special instructions
With continued fever more than 3 days and the pain more than 5 days, please contact your doctor.
Avoid the simultaneous use of paracetamol with other paratsetamolsoderzhaschimi drugs, as this may cause an overdose of paracetamol.
In applying the drug over 5-7 days should be monitored indicators of peripheral blood and functional state of the liver. Paracetamol distorts performance laboratory tests for the quantitative determination of glucose and uric acid in plasma.
Storage conditions
The reach of children at a temperature not higher than 20 ° C.
Dosing and Administration
Rectally. After a spontaneous bowel movement or enema, suppository, free from blisters and injected into the rectum. Dosage depends on the age and body weight, according to the table. A single dose of 10-15 mg / kg body weight, 2-3 times daily, 4-6 hours. The maximum daily dose of paracetamol should not exceed 60 mg / kg body weight. 1-3 4-6 mesyatsa- kg- 1 mg suppository with 50 3-12 7-10 months- kg- 1 suppository 100mg goda 1-3 1-2 11-16 kg- 100 mg suppository 3-10 17-30 years-1 kg- 250 mg suppository 10-12 31-35 years-2 kg- 250 mg suppository
Duration of treatment: 3 days as antipyretic and up to 5 days as an analgesic. The extension of the course, if necessary, after consultation with the doctor.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | STADA |











There are no reviews yet.