- No products in the cart.
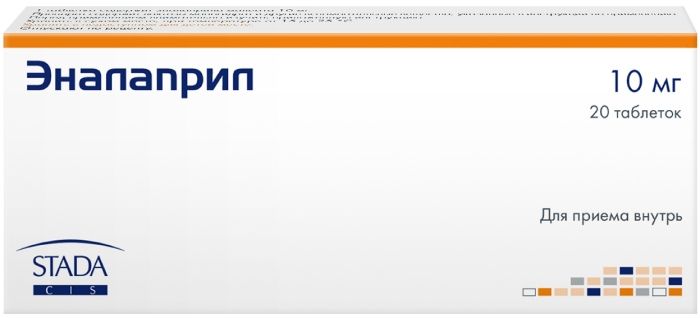
Terbinafine 250mg tabs 10 pcs vertex
$7.04
Terbinafine 250mg tabs 10 pcs vertex
Description
Composition
Active substance:
1 tablet contains: terbinafine hydrochloride in terms of 0.25 g terbinafine-
Excipients:
Microcrystalline cellulose – 0.08g, giproloza (hydroxypropyl) – 0.025 g, croscarmellose sodium – 0,025 g, the colloidal silica – 0.01g, calcium stearate – 0.005 g Lactose Monohydrate – to yield tablets each weighing 0.5 g
Description:
Round Valium tablets of white or white with a yellowish shade with a facet and Valium.
Product form:
Tablets of 250 mg.
At 7 or 10 tablets in blisters of PVC film and aluminum foil. 1, 2 or 4 blisters and 7 tablets or 1, 2 or 3 blisters with 10 tablets together with instructions for use in a stack of cardboard.
Contraindications
Increased sensitivity to terbinafine or other components of the formulation; or chronic active liver disease; chronic renal failure (less than 50 ml / min creatinine clearance); infancy (up to 3 years) and weighing up to 20 kg (for a given dosage form); lactation; lactase deficiency, lactose intolerance, malabsorption glyukozogalaktoznaya.
Caution must be exercised: renal failure (creatinine clearance greater than 50 mL / min); alcoholism; suppression of bone marrow hematopoiesis; tumors; metabolic diseases; occlusive vascular diseases of the extremities; cutaneous lupus erythematosus or systemic lupus erythematosus.
Dosage
250 mg
Indications
Fungal diseases of the skin and the nail (onychomycosis) due Trychophyton spp. (T. rubrum, T. mentagrophytes, T. verrucosum, T. tonsurans, T. violacium), Microsporum spp. (M. canis, M. gypseum), and Epidermophyton floccosum; scalp mycosis (trichophytosis, mikrosporiya); heavy, common ringworm smooth skin of the trunk and limbs requiring systemic treatment; candidiasis skin and mucous membranes.
Interaction with other drugs
Inhibits isozyme SYP2D6 and disrupts the metabolism of drugs such as tricyclic antidepressants and selective serotonin reuptake inhibitors (e.g., desipramine, fluvoxamine), beta-blockers (metoprolol, propranolol), antiarrhythmics (flecainide, propafenone), monoamine oxidase B inhibitors (e.g., selegiline ) and antipsychotics (e.g., chlorpromazine, haloperidol) means.
Medications-cytochrome P450 isoenzyme inducers (e.g. rifampicin) may accelerate metabolism and excretion from the body terbinafine. Medications inhibitors isozymes of cytochrome P450 (e.g., cimetidine) can slow metabolism and excretion from the body terbinafine. With simultaneous use of these preparty may require correction terbinafine dose.
Possible disruption of the menstrual cycle while taking terbinafine and oral contraceptives.
Terbinafine decreases the clearance of caffeine by 21% and prolongs its half-life by 31%. It does not affect the clearance of phenazone, digoxin, warfarin.
When combined with ethanol or drugs having hepatotoxic effect, there is a risk of the drug to liver.
Overdose
Symptoms: headache, dizziness, nausea, vomiting, epigastric pain, frequent urination, rash.
Treatment: The formulation for the removal event (gastric lavage, administration of activated charcoal); if necessary – symptomatic supportive therapy.
pharmachologic effect
Pharmacological group:
Antifungal agent.
Pharmacodynamics:
Terbinafine is an allylamine, which has a broad spectrum of activity against fungi which cause diseases of the skin, hair and nails, including dermatophytes. At low concentrations it has a fungicidal effect on dermatophytes Trychophyton spp. (T. rubrum, T. mentagrophytes, T. verrucosum, T. tonsurans, T. violaceum), Microsporum canis, Epidermophyton floccosum, fungi (e.g., Aspergillus, Cladosporium, Scopulariopsis brevicaulis), yeasts, primarily Candida albicans. At low concentrations of terbinafine has fungicidal activity against dermatophytes, molds and certain dimorphic fungi. Activity against yeast fungi, depending on their type, may be a fungicidal or fungistatic.
Terbinafine specifically inhibits the early stage of the biosynthesis of sterols in the fungal cell. This leads to a deficiency of ergosterol and to an intracellular accumulation of squalene, which causes death of fungal cells. Action terbinafine accomplished by inhibition of the enzyme squalene epoxidase in the cell membrane of the fungus. This enzyme does not belong to the cytochrome P450 system. Terbinafine has no influence on the metabolism of hormones or other drugs.
When receiving Terbinafine inside of the skin, hair and nails are the concentration of the drug, providing fungicidal action.
When oral administration is not effective in the treatment of pityriasis versicolor, caused by Pityrosporum ovale, Pityrosporum orbiculare (Malassezia furfur).
Pharmacokinetics:
After receiving a single oral dose of terbinafine 250 mg its maximum concentration is reached after 2 hours and the plasma is (Cmax) – 0,97 g / ml. poluabsorbtsii period of 0.8 hour period poluraspredeleniya – 4.6 hours. Communication with plasma proteins – 99%. When concomitantly with food preparation dose adjustment is required.
Terbinafine rapidly penetrates the skin and accumulate in the sebaceous glands. High concentrations are in the hair follicles and the hair after a few weeks of treatment penetrates the nail plate. Is accumulated in the stratum corneum (the concentration increased 10-fold on day 2 after ingestion of 250 mg, is 70 times – 12 days) and nails (diffusion speed exceeds nail growth rate) at concentrations providing fungicidal activity.
Terbinafine is metabolized in the liver to inactive metabolites. half-life is 16-18 hours. Terminal phase half-life – 200-400 hours. Write mainly kidneys (70%) as metabolites, as well as through the skin. Any evidence of drug accumulation in the body is not there. Excreted in breast milk. There were no changes in the equilibrium concentration of terbinafine in the plasma depends on the age, but in patients with impaired renal or hepatic function may be slow rate of excretion of the drug, which leads to higher concentrations of terbinafine blood.
Pregnancy and breast-feeding
Since safety studies terbinafine in pregnant women have not been conducted, the drug should not be administered during pregnancy. Terbinafine is excreted in breast milk, so its purpose is contraindicated during breast-feeding.
Conditions of supply of pharmacies
On prescription.
side effects
Frequency: very often – 1/10 more often – more and less than 1/100 1/10 infrequently – more and less than 1/100 to 1/1000, rarely – more and less than 1/1000 1/10000, very rarely – less 1/10000, including isolated cases. From the digestive system: very often – a feeling of fullness, loss of appetite, indigestion, nausea, abdominal pain, diarrhea; rarely – liver dysfunction; very rarely – hepatic failure, including death. From the side of hematopoiesis: rarely – neutropenia, agranulocytosis, thrombo-cytopenia, pancytopenia. Allergic reactions: very rarely – anaphylactoid reactions (including angioneurotic edema). From the nervous system: often – headache; infrequently – taste disturbance, including ageusia. For the skin: very often – skin reactions (including rash, urticaria); very rarely – Stevens-Johnson syndrome, toxic epidermal necrolysis, psoriasis-like rash, worsening of existing psoriasis, alopecia. On the part of the musculoskeletal system: very often – arthralgia, myalgia.
Other: very rarely – fatigue; cutaneous lupus erythematosus, SLE or aggravation.
special instructions
Irregular use or premature termination of treatment increases the risk of relapse.
The duration of treatment may be influenced by factors such as the presence of concomitant diseases, a condition when the nail onychomycosis in the beginning of treatment.
If after 2 weeks of treatment of skin infection is not marked improvement, it is necessary to re-determine the causative agent and its sensitivity to the drug.
Systemic application in onychomycosis is justified only in case of total destruction of most of the nail, the presence of pronounced subungual hyperkeratosis, inefficiency prior local therapy.
In the treatment of onychomycosis clinical response, laboratory confirmed, usually occurs several months after mycological cure and cessation of treatment course, due to the speed of regrowth of healthy nail.
Removing the nail plate in the treatment of onychomycosis of hands for 3 weeks and onychomycosis stop for 6 weeks is required.
In the presence of liver disease terbinafine clearance may be reduced.
During treatment necessary to monitor indicators of the activity of “liver” transaminases in blood serum.
In rare cases, after 3 months of treatment there is cholestasis and hepatitis. When signs of liver dysfunction (weakness, persistent nausea, loss of appetite, excessive abdominal pain, jaundice, dark urine or stool discolored) drug should be discontinued.
Appointment of terbinafine psoriasis patients requires caution, because In very rare cases, terbinafine can cause exacerbation of psoriasis.
In the treatment of terbinafine should comply with the general rules of hygiene to prevent the possibility of re-infection through underwear and shoes. During the treatment (2 weeks) and at the end it is necessary to produce the antifungal treatment of shoes, socks and stockings.
Impact on the management of vehicles and mechanisms
No data on the effect of Terbinafine on the ability to drive vehicles and mechanisms.
Storage conditions
In a dry, dark place at a temperature not higher than 25 C. Keep out of reach of children.
Dosing and Administration
adults:
Inside, after a meal. The usual dose: 250 mg 1 time per day.
Children over 3 years:
When a body weight of from 20 to 40 kg – 125 mg 1 time per day.
When a body weight of over 40 kg – 250 mg 1 time per day.
Duration of treatment and dosage regimen set individually and depend on the process of localization and severity of the disease.
Onychomycosis: The duration of treatment on average 6-12 weeks. In lesions nails of fingers and toes (except for the big toe), a young age or length of treatment of the patient may be less than 12 weeks. Infection of the big toe is usually sufficient 3-month course of treatment.
Some patients who have reduced the rate of growth of nails may require longer treatment.
Fungal infections of the skin: the duration of treatment with interdigital, plantar or type “socks” localization of infection is 2-6 weeks; in mycosis other areas of the body: lower leg – 2-4 weeks, body – 2-4 weeks; in mycosis caused by fungi of the genus Candida – 2-4 weeks; in mycosis of the scalp, caused by fungi of the genus Microsporum – more than 4 weeks.
The duration of treatment of fungal infections of the scalp is approximately 4 weeks after infection Microsporum canis – can be more prolonged.
Elderly patients drug is prescribed the same dose as adults.
Patients with hepatic or renal insufficiency – 125 mg 1 time per day.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | VERTEX |











There are no reviews yet.