- No products in the cart.
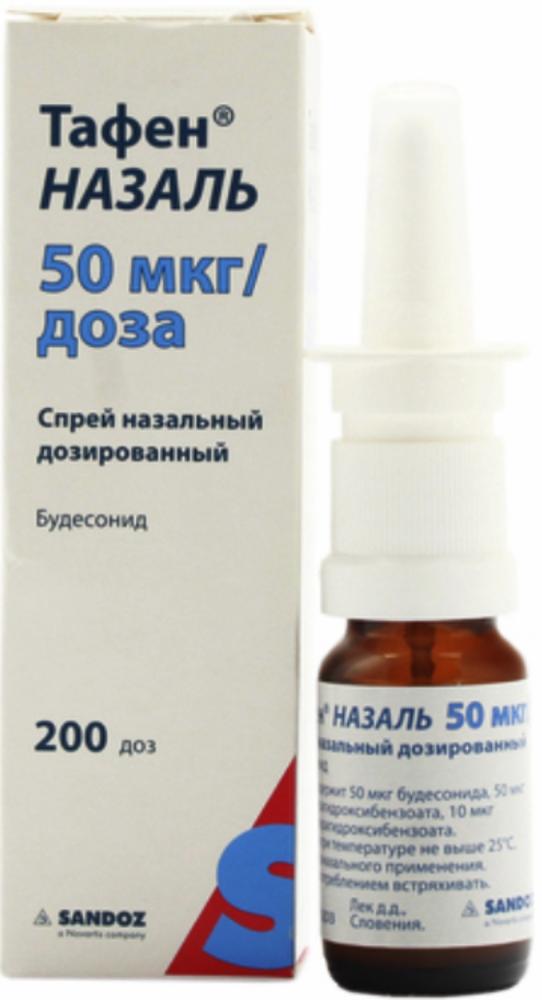
Tafen Nazal Spray Nazal. dosed 50mkg / dose 200doz 10ml vial with a metering device control
$7.45
Tafen Nazal Spray Nazal. dosed 50mkg / dose 200doz 10ml vial with a metering device control
Description
Composition
Active substance:
1 dose spray contains: 50.0 .mu.g budesonide.
Excipients:
Methyl parahydroxybenzoate, propyl parahydroxybenzoate, microcrystalline cellulose and carmellose sodium, polysorbate-80, simethicone emulsion, propylene glycol, sucrose, disodium edetate hydrochloric acid, water.
Description:
White or almost white homogeneous suspension.
Product form:
Nasal spray dosage 50 .mu.g / dose
To 10 ml in a dark glass vial of a mechanical metering device with a nozzle for the nose with the tip closed by a protective cap.
One vial with instructions for medical application is placed in a cardboard box.
Contraindications
Hypersensitivity to budesonide or excipients of the formulation; active TB disease of the lungs; Children up to age 6 years.
Precautions: fungal and viral infections of the respiratory tract, the latent form of tuberculosis (need to monitor the patient’s condition and specific therapy), recent surgery in the nasal cavity, the recent nose injury, neurotropic viral infections (herpes infections, including shingles), pregnancy, cirrhosis, glaucoma, hypothyroidism.
Dosage
50 ug / dose
Indications
Prevention and treatment of seasonal and perennial allergic rhinitis; prevention and treatment of vasomotor rhinitis; nasal polyps.
Interaction with other drugs
For budesonide did not show interactions with drugs used for the treatment of rhinitis.
The metabolism of budesonide is playing a major role isoenzyme CYP3A4. Inhibitors of this isoenzyme, such as ketoconazole and itraconazole can increase the risk of systemic effects of budesonide several times. It is necessary to avoid the simultaneous use of budesonide with these drugs. If you want to budesonide therapy and these drugs, their use should be as separated in time. You may need to reduce the dose of budesonide.
Simultaneous use of the drug Tafen® Nazal with such potential inhibitors isoenzyme CYP3A4, troleandomycin as cyclosporin and may lead to a significant increase in the concentration of budesonide in the plasma.
Simultaneous use of the drug Tafen® Nazal with inducers of microsomal oxidation (phenobarbital, phenytoin, rifampin) may decrease the effectiveness of the former.
Methandienone, estrogens and hormonal contraceptives increase the effect of budesonide. However, in the combined use of budesonide and low-dose combined oral contraceptives such an effect was not observed.
Overdose
Accidental overdose of the drug Tafen® Nazal in nasal spray dosage form dosing does not cause any obvious symptoms.
Acute overdose is unlikely.
With prolonged use of high doses, as well as the simultaneous reception of other glucocorticosteroids may appear in Cushing’s symptoms. In this case, the drug should be discontinued gradually reducing the dose.
pharmachologic effect
Pharmacological group:
Glucocorticosteroids for local use.
Pharmacodynamics:
Budeconid is a synthetic glucocorticosteroids having pronounced anti-inflammatory, anti-allergic action.
When used in therapeutic doses almost no resorptive action. Not mineralocorticoid activity, is well tolerated for prolonged treatment. The preparation has an inhibitory effect on the release of inflammatory mediators, increases the synthesis of anti-inflammatory proteins, reduces the number of mast cells and eosinophilic granulocytes, neutrophils prevents boundary distance, reduces exudation of inflammatory and cytokine production. Budesonide reduces the release of toxic proteins from eosinophils, free radicals from the macrophages and lymphokines from lymphocytes. It also reduces the binding of adhesion molecules to endothelial cells, thus, reducing the influx of leukocytes to the site of allergic inflammation. Budesonide increases the amount of alpha-adrenergic receptors on the membrane surface of vascular smooth muscle cells, thus increasing sensitivity to decongestants. The drug inhibits phospholipase A2 activity, which leads to the inhibition of synthesis of prostaglandins, leukotrienes and platelet activating factor, inducing an inflammatory response. Budesonide also inhibited the synthesis of histamine, which leads to a decrease of its level in mast cells. Improvement observed on the second or third day after the start of treatment.
Tafen® Nazal reduces the severity of symptoms for allergic rhinitis, suppresses early and late phase allergic reactions and reduces inflammation in the upper airways.
Pharmacokinetics:
After topical application of 400 .mu.g budesonide maximum concentration Cmax plasma levels achieved after about 30 minutes and was 1 nmol / l. Only about 20% of the dose of the drug administered Tafen® Nazal reaches the systemic circulation. Due to the good tissue distribution and binding to plasma proteins, volume of distribution is 301 liters. Binding to plasma proteins, mainly albumin is 86-90%.
Systemic bioavailability of budesonide is low, since more than 90% of absorbed drug is inactivated during the “first pass” through the liver. Glucocorticoid activity of metabolites does not exceed 1%. Metabolites derived mainly kidneys (70%) and in the intestine (10%). The half-life (T1 / 2) was 2 h.
Pregnancy and breast-feeding
Pregnancy
Prospective epidemiological studies, as well as the use of budesonide in the post-marketing period, all over the world showed no increased risk of congenital malformations due to the use of inhaled and nasal forms of budesonide during early pregnancy.
Use of the drug during pregnancy Tafen® Nazal allowed only if the expected benefit to the mother outweighs the potential risk to the fetus.
Breast-feeding
The drug passes into breast milk. However, when using therapeutic doses of budesonide for the child who is breastfed is expected. Budesonide may be applied during breastfeeding.
Supportive asthma inhaled budesonide therapy (at a dosage of 200 mg or 400 mg) in lactating women lead to a minimum the effects of the drug on a child who is breastfed.
The pharmacokinetic studies estimated daily dose to a child, was 0.3% of the daily dose of the parent, and the average concentration in the blood plasma of the child – 1/600 of the concentration observed in the plasma, maternal blood. The concentration of budesonide in all blood plasma samples of children were below the detection limit.
Based on the data obtained for inhaled budesonide and the fact that budesonide has linear pharmacokinetic properties when using therapeutic doses nasally, by inhalation, orally, rectally, possible effects on a child who is breastfed unlikely.
Conditions of supply of pharmacies
On prescription.
side effects
According to the World Health Organization (WHO) adverse reactions are classified according to their rate of development as follows: very common (> 1/10), common (> 1/100,
special instructions
It is recommended to avoid contact with the drug Tafen® Nazal eyes!
When long-term administration of high doses of budesonide can not eliminate the risk of systemic manifestations of the drug. The probability of systemic effects are much lower than with oral corticosteroids. There may be individual differences in the effects of such development. Potential systemic effects may include Cushing’s syndrome, symptoms kushingoida, adrenal suppression, growth retardation in children and adolescents, decrease in bone mineral density, the symptoms of hypothyroidism or Cushing’s, cataracts and glaucoma. Also nausea, altered taste, difficulty swallowing, anosmia, palpitations, nasal congestion, dizziness, headache, sore throat, conjunctival hyperemia, myalgia, drowsiness, cough. Less commonly, there may be deviation of psychological and behavioral issues, including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children).
When switching from treatment with systemic corticosteroids nasal spray for treatment in connection with the risk of adrenal insufficiency, caution is required for a recovery period of the hypothalamic-pituitary-adrenal axis (HPA axis) and the gradual removal of the drug (gradual reduction in the dose normalization for HPA axis function). At the stage of reducing the dose in some patients may appear the withdrawal symptoms of systemic glucocorticosteroids, such as muscle pain and / or joint pain, lethargy and depression. In identifying these symptoms may require a temporary increase in the dose of systemic glucocorticosteroids, and subsequently, further cancellation at a slower pace.
Since steroids retard wound healing, caution should be exercised when administering the drug Tafen® Nazal patients recently suffered injury or nose surgery. For full therapeutic effect required to regularly use the drug for allergic rhinitis. During prolonged therapy with Tafen® Nazal necessary to assess the state of the mucous membrane of the nose every 6 months.
cases of growth retardation in children have been reported treated with steroids for nasal administration in therapeutic doses. With prolonged use of glucocorticoids for nasal use in children is recommended to control the growth dynamic. When decelerating pediatrician growth must rethink the way the drug to reduce the dose and the transition to the minimum therapeutic dose, in which it is possible to control the symptoms of the disease.
The use of nasal corticosteroids at doses higher than the recommended may lead to a significant inhibition of adrenal function. In this case, to consider the need for further use of systemic corticosteroids in reducing the period of adrenal function or elective surgery.
Abnormal liver function may result in reduced excretion of corticosteroids and enhance the probability of systemic exposure.
Since adrenal function may be reduced, adrenocorticotropic hormone stimulation test to detect operation pituitary disorders may give incorrect (too low) results.
Caution should be exercised when using the drug Tafen® Nazal in patients with latent tuberculosis, as well as fungal infections and viral etiology.
Patients taking the drug Tafen® Nazal, should be informed that the full effect develops within a few days after the start of the drug.
The use of budesonide has no effect on the ability to drive vehicles, machinery.
Storage conditions
Store at a temperature not higher than 25 C. Keep out of reach of children !.
Dosing and Administration
Adults and children over 6 years: initially 2 doses of 50 mcg of budesonide in each nostril 2 times a day. The usual maintenance dose is 1 dose in each nostril two times a day, or 2 doses in each nostril once a day 1, the morning. The maintenance dose should be the lowest effective dose of relieving symptoms of rhinitis.
The maximum single dose of 200 micrograms (100 micrograms into each nostril), the maximum daily dose of 400 ug for no more than 3 months.
For full therapeutic effect requires regular and correct application. If receiving a dose is missed, it should be taken as soon as possible, but not less than 1 hour prior to the next regular dose.
The nozzle and the cap should be cleaned regularly.
Carefully remove the nozzle and the cap, warm rinse, and rinse with cold water and allow to air dry. Carefully place the nozzle in its place and close the cap.
If the hole has accumulated a dried preparation, hold the nozzle in the vessel with warm water, then wash as described above. Do not clean the nozzle opening with a needle or other sharp objects.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | Sandoz RX |











There are no reviews yet.