- No products in the cart.
Singulair tabs Gesves. 4mg 14 pc

Solgar lozenges 50 pcs Flavio zinc
$14.54
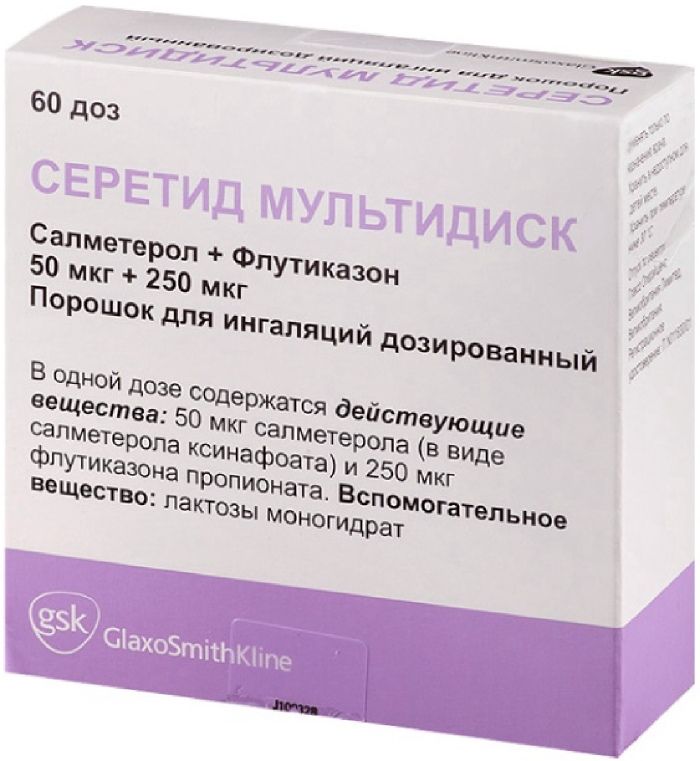
Seretide multidisk powder ing.dozir. 50mkg / 250mkg 60doz with inhaler
$33.31
$33.06
Singulair tabs Gesves. 4mg 14 pc
Description
Composition
Active substance:
1 tablet comprises montelukast sodium 4.16 mg (equivalent to 4.0 mg free acid).
Excipients:
Mannitol 161.08 mg Cellulose microcrystalline 52.8 mg, giproloza (hydroxypropylcellulose), 7.2 mg red ferric oxide 0.36 mg, 7.2 mg croscarmellose sodium, cherry flavor 3.6 mg, 1.2 mg of aspartame, magnesium stearate 2.4 mg.
Description:
Pink, oval, biconvex tablets with embossed inscription «SINGULAIR» on one side and «MSD 711 ‘on the other side.
Product form:
Chewable tablet 4 mg.
7 chewable tablets in the blister 4 mg PVC-Al foil. 1, 2 or 4 in the blister cardboard pack with instructions for use.
Contraindications
Hypersensitivity to any component of the drug.
Children under 2 years old.
Phenylketonuria.
Dosage
4 mg
Indications
Prevention and long-term treatment of asthma in children aged 2 to 5 years, including the prevention of daytime and nighttime symptoms, the treatment of asthma in patients with hypersensitivity to aspirin and prevention of bronchospasm caused by exercise.
Relief of daytime and nighttime symptoms of seasonal and / or perennial allergic rhinitis in children aged 2 to 5 years.
Interaction with other drugs
Singulyar® The drug may be administered together with other drugs that are usually used for the prevention and long term treatment of asthma and / or allergic rhinitis. The recommended therapeutic dose of montelukast no clinically significant effect on the pharmacokinetics of the following drugs: theophylline, prednisone, prednisolone, oral contraceptives (ethinyl estradiol / norethisterone 35/1), terfenadine, and warfarin digoxin.
Montelukast AUC value decreases while receiving phenobarbital about 40%, but it does not require changes in the dosing regimen Singulyar® preparation.
In in vitro studies have shown that montelukast inhibited CYP 2C8 isoenzyme of cytochrome P450, but in the study of drug interactions in vivo montelukast and rosiglitazone (metabolized involving CYP 2C8 isoenzyme of cytochrome P450) it was shown that not montelukast inhibited isoenzyme CYP 2C8. Thus, not supposed to influence montelukast on CYP 2C8-mediated metabolism of drugs (e.g., paclitaxel, rosiglitazone, repaglinide).
Studies in vitro have shown that montelukast is a substrate isozymes CYP 2C8, 2C9 and 3A4. These clinical studies of drug interactions in relation montelukast and gemfibrozil (an inhibitor of both CYP 2C8, and 2C9) demonstrated that gemfibrozil effect increases the systemic exposure of Montelukast 4.4 times. Co-administration of itraconazole, a potent inhibitor of CYP 3A4, with gemfibrozil and montelukast did not lead to further increase the effect of systemic exposure of Montelukast.
Effect of gemfibrozil on systemic exposure of Montelukast can not be considered clinically significant on the basis of safety when used at doses higher than the approved dose of 10 mg for adults (for example, when used in a dose of 200 mg / day for adult patients for 22 weeks, and up to 900 mg / day for about one week, no clinically significant adverse effects). Therefore, when co-administered with gemfibrozil montelukast dose adjustment is required. By in vitro studies results not expected clinically significant drug interactions with other known inhibitors of CYP 2C8 (e.g., trimethoprim).
In addition, co-administration of montelukast with itraconazole alone did not lead to a significant increase in the effect of the systemic exposure of montelukast.
Combined treatment with bronchodilators
The drug is justified Singulyar® addition to bronchodilators alone, if they do not provide adequate control of asthma. Upon reaching the therapeutic effect of the treatment with Singulyar® can start a gradual reduction in the dose of bronchodilators.
Combined treatment with inhaled glucocorticosteroids
Treatment with Singulyar® provide additional therapeutic benefit to patients applying inhaled glucocorticosteroids. Upon reaching stabilization can start a gradual reduction of the dose of glucocorticosteroid under the supervision of a physician. In some cases a complete abolition of inhaled glucocorticosteroids, but a sharp substitution of inhaled glucocorticosteroids on Singulyar® drug is not recommended.
Overdose
No specific information on the treatment of drug overdose Singulyar®. Overdosage symptoms were not observed in clinical studies of long-term (22 weeks) treatment of adult patients with asthma with daily doses of the drug Singulyar® to 200 mg, either in the course of short (about 1 week) clinical studies with daily doses up to 900 mg.
There have been cases of acute overdose space (receiving at least 1,000 mg per day) in the preparation Singulyar® Post-registration period and during clinical studies in adults and children. Clinical and laboratory data showed a comparable safety profile of the drug Singulyar® in children, adults and elderly patients.
The most common side effects are thirst, drowsiness, vomiting, agitation, headache, and abdominal pain. These side effects are consistent with the safety profile of the drug Singulyar®.
Treatment in the event of acute overdose is symptomatic.
Data on the effectiveness of peritoneal dialysis or hemodialysis montelukast no.
pharmachologic effect
Pharmacological group:
Antibronhospasticheskoe anti-inflammatory agent, a leukotriene-receptor blocker.
Pharmacodynamics:
Cysteinyl leukotrienes (LTC4, LTD4, LTE4) are potent mediators of inflammation – eicosanoids, which are released by different cells including mast cells and eosinophils. These important proastmaticheskie mediators bind to cysteinyl leukotriene receptor. Cysteinyl leukotriene receptor type 1 (CysLT1-receptors) are present in the human airway (including, in the cells of the bronchial smooth muscle, macrophages) and other proinflammatory cells (including eosinophils and certain myeloid stem cells). Cysteinyl leukotrienes are correlated with the pathophysiology of asthma and allergic rhinitis. In asthma, leukotriene-mediated effects include bronchoconstriction, increased mucus secretion, and increased vascular permeability increase in the number of eosinophils. In allergic rhinitis after allergen exposure is released cysteinyl leukotrienes from inflammatory cells of nasal mucosa during the early and late phase allergic reaction, which manifests symptoms of allergic rhinitis. When intranasal sample with cysteinyl leukotrienes was shown to increase resistance of pneumatic nasal passages and nasal obstruction symptoms.
Montelukast – highly active when administered drug, which significantly improves indices of inflammation in bronchial asthma. According to the biochemical and pharmacological analysis of Montelukast with high affinity and selectivity binds CysLT1-receptors without interacting with other pharmacologically important receptors in the airways (such as prostaglandin, cholinergic or adrenergic beta-receptors). Montelukast inhibits the physiological effect of cysteinyl leukotrienes LTC4, LTD4 and LTE4 by binding to CysLT1-receptors without exerting stimulatory effect on these receptors.
Montelukast inhibits CysLT-receptors in the respiratory tract, as evidenced by the ability to block the development of bronchospasm in response to the inhalation of LTD4 in patients with bronchial asthma patients. Dose of 5 mg is sufficient for relief of bronchospasm induced by LTD4.
Montelukast causes bronchodilation within 2 hours after ingestion, and can complement bronchodilation caused by beta2-agonists.
Use of montelukast at doses exceeding 10 mg per day, taken singly, does not increase the efficacy of the drug.
Pharmacokinetics:
Suction
Montelukast is rapidly and almost completely absorbed after oral administration. In adults in the fasting coated tablets, 10 mg of the maximum concentration (Cmax) is reached after 3 chasa (Tmah). The mean oral bioavailability is 64%.
Food intake does not affect the Smah in plasma and bioavailability of the drug.
Distribution
Montelukast binds to plasma proteins by more than 99%. The volume of distribution of montelukast in a state of equilibrium concentration averages 8-11 liters.
Studies with radiolabeled montelukast conducted on rats indicate a minimal penetration of the blood brain barrier. Furthermore, the concentration of labeled drug by 24 hours after administration were minimal in all other tissues.
Metabolism
Montelukast is extensively metabolised. In the study of the therapeutic dose in adults and children concentration of metabolites of montelukast in the equilibrium state is not detected in the plasma.
Studies in vitro using human liver microsomes revealed that participate in the metabolism of montelukast cytochrome P450 isoenzymes: 3A4, 2S8 and 2S9. According to studies conducted in vitro in human liver microsome, Montelukast therapeutic blood plasma concentration does not inhibit cytochrome P450 isoenzymes: 3A4, 2C9, 1A2, 2A6, 2C19 and 2D6.
breeding
The plasma clearance of montelukast in healthy adults is 45 ml / min.
After ingestion of radiolabelled montelukast 86% of the amount excreted in the feces within 5 days and less than 0.2% – in the urine, which confirms that montelukast and its metabolites are excreted almost exclusively with bile.
The half-life of montelukast in young healthy adults is between 2.7 and 5.5 hours. Pharmacokinetics montelukast retains substantially linear ingestion doses greater than 50 mg. When receiving montelukast in the morning and evening differences pharmacokinetics was observed. Upon receiving the 10 mg montelukast 1 once daily observed moderate (about 14%) cumulation of active substance in plasma.
Pharmacokinetics in different patient groups
Floor
The pharmacokinetics of montelukast in women and men is similar.
elderly patients
In single dose administration of 10 mg montelukast pharmacokinetic profile and bioavailability similar in elderly and young patients. The half-life of montelukast plasma slightly longer in the elderly. Correction dose in the elderly is not required.
Race
There were no differences in clinically relevant pharmacokinetic effects in patients of different races.
Liver failure
Patients with liver failure mild to moderate severity and clinical manifestations of cirrhosis marked slowing metabolism of montelukast, accompanied by increase in the area under the curve “concentration-time» (AUC) of about 41% after a single dose of the drug at a dose of 10 mg. Withdrawal of montelukast in these patients, a slight increase compared to healthy subjects (mean half-time – 7.4 hours). Changing the dose of montelukast in patients with hepatic insufficiency, mild to moderate severity is not required. Information on the nature of the pharmacokinetics of montelukast in patients with severe hepatic insufficiency (more than 9 points on a scale Child-Pugh) do not.
kidney failure
Because montelukast and its metabolites are not excreted in the urine, the pharmacokinetics of montelukast in patients with renal insufficiency has not been assessed. Correction dose for this patient group is not required.
Pregnancy and breast-feeding
Singulyar® clinical trials of the drug in pregnant women have been conducted. Singulyar® drug should be used during pregnancy and lactation only if the expected benefit to the mother outweighs the potential risk to the fetus or child. During post-marketing use of the drug Singulyar® reported on the development of birth defects of extremities in newborns whose mothers took the drug during pregnancy Singulyar®. Most of these women were also taking other medications for the treatment of asthma during pregnancy.
The causal relationship between the intake of the drug and the development Singulyar® birth defects limb has not been established.
It is unknown whether Singulyar® drug is excreted in breast milk. Because many drugs are excreted in breast milk, it is necessary to consider the appointment of the drug Singulyar® lactating mothers.
Conditions of supply of pharmacies
On prescription.
side effects
In general Singulyar® preparation well tolerated. Side effects are usually mild and usually did not require discontinuation of therapy. The overall incidence of side effects in the treatment of drug Singulyar® comparable to their frequency in placebo.
Children aged 2 to 5 years with asthma
In clinical studies, the drug Singulyar® took part in 573 patients aged 2 to 5 years. In the 12-week, placebo-controlled clinical study, only one adverse event (AEs), estimated to be related to administration of the drug, observed at> 1% of patients taking the drug Singulyar®, and more often than in patients treated with placebo was thirst. Differences in the frequency of adverse events between the two treatment groups were not statistically significant.
In total, 426 patients aged from 2 to 5 years were treated with drug
Singulyar® for at least 3 months 230 – for 6 months or longer, and 63 patients – for 12 months or longer. With longer treatment profile of adverse events did not change.
Children aged 2 to 14 years of age with seasonal allergic rhinitis
The 2-week, placebo-controlled clinical study using a drug
Singulyar® for the treatment of seasonal allergic rhinitis received 280 patients between the ages of 2 and 14 years. The drug is taken by the patient Singulyar® 1 time per day in the evening and was generally well tolerated, with a safety profile of the drug was similar to placebo safety profile. In this clinical trial, adverse events were not registered, which would be regarded as being associated with taking the drug were observed to have> 1% of patients taking the drug Singulyar®, and more frequently than in patients receiving placebo.
Children aged 6 to 14 years with asthma
The safety profile of the drug in children was generally similar to the safety profile in adults and comparable to placebo safety profile.
In an 8-week, placebo-controlled clinical study, the only adverse events judged to be drug-related, observed in> 1% of patients taking the drug Singulyar®, and more frequently than in patients treated with placebo was headache. The difference in frequency between the two treatment groups was not statistically significant.
The estimated rate of growth of the safety profile trials in patients in this age group corresponds to the previously described safety profile of the drug Singulyar®.
A more long-term treatment (more than 6 months.), The profile of adverse events did not change.
Adults and children 15 years of age and older with asthma
Two 12-week, placebo-controlled clinical study with a similar design, the only AEs assessed as related to the administration of the drug observed at> 1% of patients taking the drug Singulyar®, and more often than in patients treated with placebo were pain in the abdomen and headache. Differences in frequency data AEs between the two treatment groups were not statistically significant. A more long-term treatment (for 2 years) profile of adverse events did not change.
Adults and children aged 15 years and older with seasonal allergic rhinitis
The drug is taken by the patient Singulyar® 1 times a day, morning or evening, and was generally well tolerated, with a safety profile of the drug was similar to placebo safety profile.
In placebo-controlled clinical trials, adverse events were not registered, which would be regarded as being associated with taking the drug were observed to have> 1% of patients taking the drug Singulyar®, and more frequently than in patients receiving placebo. In a 4-week placebo-controlled clinical study, the safety profile of the drug was similar to that of a 2-week studies. The incidence of somnolence while taking the drug in all studies was the same as with placebo.
Adults and children aged 15 years and older with perennial allergic rhinitis
The drug is taken by the patient Singulyar® 1 times a day, and was generally well tolerated.
The safety profile of the drug was similar to the safety profile observed in patients with seasonal allergic rhinitis and placebo. In these clinical studies, adverse events were not registered, which would be regarded as being associated with taking the drug would have been observed in> 1% of patients taking the drug
Singulyar®, and more frequently than in patients receiving placebo. The incidence of sleepiness when receiving the preparation was the same as that of placebo.
A pooled analysis of clinical trials
Generalized analysis was conducted 41 placebo-controlled clinical trial (35 trials involving patients aged 15 years or older; 6 trials involving patients aged 6 to 14 years) using approved methods for assessing suicidality. Among the 9929 patients treated with the drug Singulyar® and 7780 patients taking placebo in these studies, it was diagnosed one patient with suicidal thoughts in patients taking the drug Singulyar®. Ни в одной из групп лечения не было совершено ни одного самоубийства, суицидальной попытки или других подготовительных действий, указывавших на суицидальное поведение.
Отдельно был проведен обобщенный анализ 46 плацебо-контролируемых клинических исследований (35 исследований с участием пациентов в возрасте 15 лет и старше; 11 исследований с участием пациентов в возрасте от 3 месяцев до 14 лет) для оценки неблагоприятных поведенческих эффектов (НПЭ). Среди 11673 пациентов, принимавших в этих исследованиях препарат Сингуляр®, и 8827 пациентов, принимавших плацебо, процент пациентов, имеющих как минимум один НПЭ, составил 2,73% среди принимавших препарат
Сингуляр® и 2,27% – среди принимавших плацебо; отношение шансов составило 1,12 (95% доверительный интервал [0,93; 1,36]).
За время пострегистрационного применения препарата было сообщено о следующих выявленных НЯ: инфекционные и паразитарные заболевания: инфекции верхних дыхательных путей; нарушения со стороны крови и лимфатической системы: повышение склонности к кровотечениям, тромбоцитопения; нарушения со стороны иммунной системы: реакции гиперчувствительности, в том числе анафилаксия, очень редко (
special instructions
Эффективность препарата Сингуляр® для перорального приема в отношении лечения острых приступов бронхиальной астмы не установлена, поэтому препарат Сингуляр® в таблетках не рекомендуется назначать для лечения острых приступов бронхиальной астмы. Пациентам должны быть даны инструкции всегда иметь при себе препараты экстренной помощи для купирования приступов бронхиальной астмы (ингаляционные бета-2-агонисты короткого действия).
Не следует прекращать прием препарата Сингуляр® в период обострения астмы и необходимости применения препаратов экстренной помощи для купирования приступов (ингаляционных бета-2-агонистов короткого действия).
Пациенты с подтвержденной аллергией к ацетилсалициловой кислоте и другим нестероидным противовоспалительным препаратам (НПВП) не должны принимать эти препараты в период лечения препаратом Сингуляр®, поскольку препарат Сингуляр®, улучшая дыхательную функцию у больных аллергической бронхиальной астмой, тем не менее не может полностью предотвратить вызванную у них НПВП бронхоконстрикцию.
Дозу ингаляционных глюкокортикостероидов, применяемых одновременно с препаратом
Сингуляр®, можно постепенно снижать под наблюдением врача, однако резкой замены ингаляционных или пероральных глюкокортикостероидов препаратом Сингуляр® проводить нельзя. У пациентов, принимавших препарат Сингуляр®, были описаны психоневрологические нарушения (см. раздел «Побочное действие»). Учитывая, что эти симптомы могли быть вызваны другими факторами, неизвестно, связаны ли они с приемом препарата Сингуляр®.
Врачу необходимо обсудить данные НЯ с пациентами и/или их родителями/опекунами.
Пациентам и/или их родителям/опекунам необходимо объяснить, что в случае появления подобных симптомов необходимо сообщить об этом лечащему врачу.
В редких случаях пациенты, получавшие противоастматические препараты, включая антагонисты лейкотриеновых рецепторов, испытывали одно или несколько НЯ из ниже перечисленных: эозинофилию, сыпь, ухудшение легочных симптомов, кардиологические осложнения и/или нейропатию, иногда диагностируемую как синдром Чарджа-Стросс, системный эозинофильный васкулит. Эти случаи иногда были связаны со снижением дозы или отменой терапии пероральными глюкокортикостероидами. Хотя причинно-следственной связи этих НЯ с терапией антагонистами лейкотриеновых рецепторов не было установлено, пациентам, принимающим препарат Сингуляр®, необходимо соблюдать осторожность; у таких пациентов необходимо проводить соответствующее клиническое наблюдение.
Препарат Сингуляр® таблетки, покрытые оболочкой, 10 мг содержит лактозы моногидрат.
Пациенты с редкой формой наследственной непереносимости галактозы, врожденной недостаточностью лактазы или глюкозо-галактозной мальабсорбцией не должны принимать препарат Сингуляр® таблетки, покрытые оболочкой, 10 мг.
Применение у пожилых пациентов
Различий в профилях эффективности и безопасности препарата Сингуляр®, связанных с возрастом пациентов, не выявлено.
ВЛИЯНИЕ НА СПОСОБНОСТЬ УПРАВЛЯТЬ ТРАНСПОРТНЫМИ СРЕДСТВАМИ И РАБОТАТЬ С МЕХАНИЗМАМИ
Не ожидается, что прием препарата Сингуляр® будет влиять на способность управлять транспортными средствами и работать с механизмами. Тем не менее индивидуальные реакции на препарат могут быть различными. Некоторые побочные эффекты (такие как головокружение и сонливость), которые, как сообщалось, очень редко возникали при применении препарата
Сингуляр®, могут влиять на способность некоторых пациентов управлять транспортными средствами и работать с механизмами.
Storage conditions
Хранить при температуре 15-30 С в защищенном от влаги и света месте. Keep out of the reach of children.
Dosing and Administration
Внутрь 1 раз в сутки независимо от приема пищи. Для лечения бронхиальной астмы препарат
Сингуляр® следует принимать вечером. При лечении аллергических ринитов доза может приниматься в любое время суток по желанию пациента. Пациенты с бронхиальной астмой и аллергическими ринитами должны принимать одну таблетку препарата Сингуляр® 1 раз в сутки вечером.
Дети в возрасте от 2 до 5 лет с бронхиальной астмой и/или аллергическим ринитом
Дозировка для детей 2–5 лет составляет одну жевательную таблетку 4 мг в сутки.
Общие рекомендации
Терапевтическое действие препарата Сингуляр® на показатели, отражающие течение бронхиальной астмы, развивается в течение первого дня. Пациенту следует продолжать принимать Сингуляр® как в период достижения контроля за симптомами бронхиальной астмы, так и в периоды обострения бронхиальной астмы.
Для пожилых пациентов, пациентов с почечной недостаточностью, а также пациентов с легкими или среднетяжелыми нарушениями функции печени, а также в зависимости от пола специального подбора дозы не требуется.
Назначение препарата Сингуляр® одновременно с другими видами лечения бронхиальной астмы
Препарат Сингуляр® можно добавлять к лечению пациента бронходилататорами и ингаляционными глюкокортикостероидами (см. раздел «Взаимодействие с другими лекарственными препаратами»).
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | RX DPA |









There are no reviews yet.