- No products in the cart.
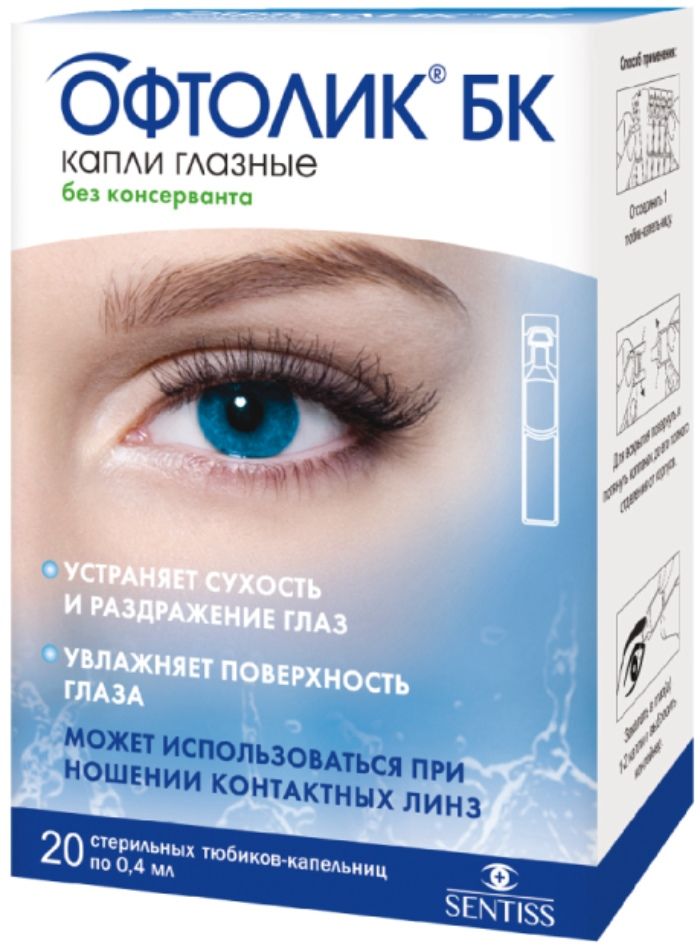
Oftolik BC drops Ch. preservative-free 0.4ml Tub-cap. 20 pc
Phenylephrine optician drops Ch. 2.5% 5ml vial-cap. Lecco / Pharmstandard
$7.66
Anastrozole tab n / 1mg film about 30 pieces of ozone
$29.31
$9.34
Oftolik BC drops Ch. preservative-free 0.4ml Tub-cap. 20 pc
SKU: 01914467678 Categories: Medicaments, Moisturizing the mucous membranes, Ophthalmology Tag: Sentis
Description
Composition
Active substance:
1 ml contains: polyvinyl alcohol 14 mg, 6 mg povidone ,.
Excipients:
Disodium edetate 1 mg, 8.46 mg sodium chloride, sodium hydroxide q.s., hydrochloric acid q.s., water for injection q.s. to 1 ml.
Description:
Transparent, colorless or slightly yellowish solution.
Product form:
Eye drops without preservatives.
In a 0.4 ml tube-dropper. 5 tubes, droppers in a plastic cartridge, enclosed in the package of laminated foil. 1, 2, 4 or 6 pack to pack carton along with instructions for use.
Contraindications
Hypersensitivity to the drug.
Pregnancy and lactation (it is impossible to assess the balance of risk to the fetus and the child and the benefit to the mother in the absence of safety data).
Indications
Syndrome of “dry” eyes.
Interaction with other drugs
Polyvinyl alcohol subjected to esterification reactions characteristic of compounds with secondary hydroxy groups. He collapses in strong acids and softens or dissolves in weak acids and alkalis. At higher concentrations the substance incompatible with inorganic salts, especially phosphates and sulphates. Sedimentation 5% polyvinyl alcohol may be caused by a reaction with phosphates. Gel formation of the PVA solution can occur in the presence of borax.
Povidone solution is compatible with many inorganic salts, natural and synthetic resins and other substances. Is reacted in solution with sulfathiazole, sodium salicylate, salicylic acid, phenobarbital, tannin and other substances. The efficacy of some preservatives, such as thiomersal, may be reduced due to the formation of complexes with povidone.
Overdose
Data on overdose by topical application no. In case of accidental admission into recommended to consult a doctor.
pharmachologic effect
Pharmacological group:
Keratoprotektor.
Pharmacodynamics:
The drug has a protective effect on the cornea at a reduced secretion of tear fluid, or by increasing the evaporation of the tear film.
Polyvinyl alcohol and povidone have lubricant properties, which reduces irritation and redness of the eye. These substances reduce the surface tension of tear film, covering the surface of the eye easily and prevent the occurrence of gap portions tear film. Polyvinyl alcohol has properties similar to those of mucin-producing glands in the conjunctival. It contributes to mitigate and smearing (wetting) the surface of the eye, increases the stability of tear film.
Pharmacokinetics:
Through the study of the bioavailability it has been found that after instillation of two drops of BC in each eye Oftolik® component concentration of drug in plasma after 4 hours after use remains lower possible limit of quantitation (10 ng / ml). This allows to conclude that the systemic absorption of the drug component to the ocular surface using a BK eyedrops Oftolik® minimal.
Pregnancy and breast-feeding
Sufficient experience of use of the drug during pregnancy and lactation is not. The use in pregnant women and nursing mothers can only be prescribed by a doctor, if the expected therapeutic effect is greater than the risk of possible side effects.
Conditions of supply of pharmacies
Without recipe.
side effects
Transient burning sensation, eye pain, eye redness, headache, redness of the skin around the eyes, blurred vision short. Allergic reactions to components of the formulation.
special instructions
Do not use the drug if the color change of the solution, or it became cloudy.
Ensure that the tip of the vial during use does not touch any surfaces. After use, close the bottle lid.
Eye drops BC Oftolik® may cause transient blurring of vision. Before you start driving or using mechanical devices must wait until the visual acuity completely recovered.
Storage conditions
In the dark place at a temperature not higher than 25 ° C. Do not freeze.
Keep out of the reach of children.
Dosing and Administration
1-2 drops 3-4 times daily in both eyes, depending on the severity of symptoms.
Before using the product, wash hands.
Shake the bottle and remove the cover. Ensure that the pipette tip does not touch the skin around the eyes or the surface of the eye to prevent infection.
Tilt the head backward, pull the lower lid, and turn the bottle drip required number of drops into the conjunctival sac.
Duration of treatment is established doctor depending on the severity of symptoms. If no relief of symptoms within 72 hours should again consult a doctor.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | Sentis |










There are no reviews yet.