- No products in the cart.
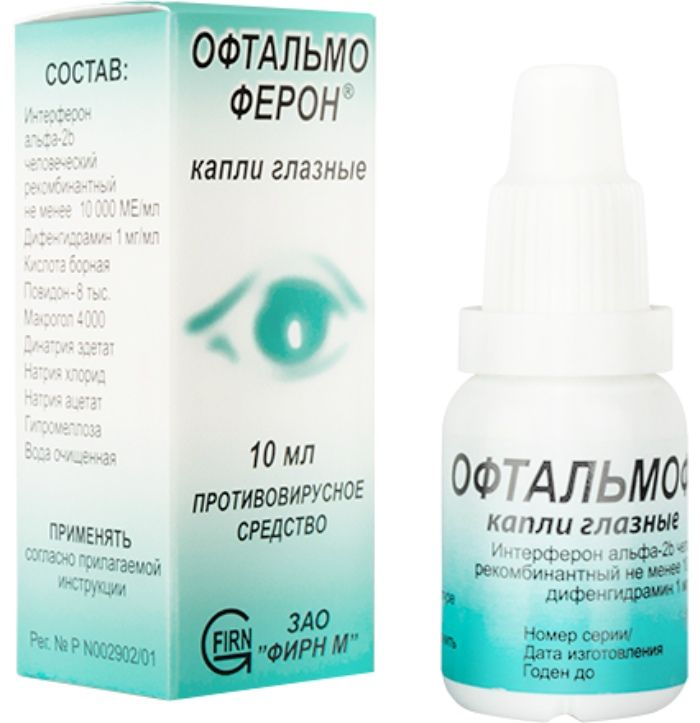
Oftalmoferon drops Ch. 10000me / ml-10ml vial cap.

Teagel Steri stock gel hygiene eyelids / eyelashes 30g
$12.34
Hydrocortisone ointment Ch. 0.5% 5 g tube
$1.01
$6.86
Oftalmoferon drops Ch. 10000me / ml-10ml vial cap.
Out of stock
Description
Composition
Active substance:
1 ml of solution contains: interferon alpha-2b, recombinant human least 10,000 ME, diphenhydramine hydrochloride (diphenhydramine) 1.0 mg.
Excipients:
Boric acid 3.1 mg Disodium edetate 0.4 mg Sodium chloride 2.2 mg sodium acetate, 7.0 mg, 3.0 mg of hypromellose, povidone-8 thousand 5 mg, 50 mg macrogol 4000, purified water to 1 ml.
Description:
The clear colorless or yellowish white with a weak solution.
Product form:
Eye drops. 5 ml and 10 ml vials with plastic dropper dispenser. 1 bottle together with instructions for use placed in a pile of cardboard.
Contraindications
Hypersensitivity of drug components.
Indications
Adenovirus, hemorrhagic (enterovirus), herpetic conjunctivitis; adenovirus, herpes (vesicular, point, tree, kartoobrazny) keratitis; herpetic stromal keratitis and corneal ulceration with no ulceration; adenovirus and herpes keratoconjunctivitis; herpetic uveitis; herpetic keratouveitis (with or without ulceration); syndrome of “dry” eyes; prevention of graft disease and preventing recurrence of herpetic keratitis after keratoplasty; Prevention and treatment of complications after excimer laser refractive surgery of the cornea.
Interaction with other drugs
The drug is compatible well with anti-inflammatory, antibacterial, corticosteroids, ocular drugs reparative – corneal regeneration stimulants and drugs slezozamestitelnoy therapy.
Overdose
Cases of overdose have been identified.
pharmachologic effect
Pharmacological group:
Antiviral combination means (+ cytokine H1-histamine receptor blocker).
Pharmacological properties:
Oftalmoferon® is a combination drug containing in its composition antiviral and immunomodulating agents – interferon alfa-2b, recombinant human, and antihistamines – diphenhydramine.
Pharmacodynamics:
Human recombinant interferon alfa-2b has a broad spectrum of antiviral activity, immunomodulatory, anti-proliferative effect. Diphenhydramine – blocker H1-histamine receptors, exerts an antiallergic effect, reduces swelling and itching of the conjunctiva.
Pharmacokinetics:
When topically applied drug is not subjected to systemic adsorption. The concentration of active substances attainable in blood is significantly below the detection limit (detection limit of interferon alpha-2b – 1.2 IU / ml) and has no clinical significance. Information about the extent of penetration of diphenhydramine in various eye tissues after topical application no.
Pregnancy and breast-feeding
Use of the drug during pregnancy and lactation is possible only by the NIJ-appointed physician, if the expected benefits outweigh the risks of complications in the fetus and newborn.
Conditions of supply of pharmacies
Without recipe.
side effects
Not noted.
special instructions
Patients who use contact lenses must bury the drug only at the removed lenses and can put them in 15-20 minutes after instillation of the preparation.
Immediately after instillation can be blurred vision, so it is recommended to start to drive vehicles or operate machinery after a few minutes after instillation of the drug.
Storage conditions
The preparation is stored in the dark place at a temperature of 2 to 8 ° C. Keep out of the reach of children.
Transportation carried by all types of covered transport at 2 to 8 ° C.
Dosing and Administration
In viral eye damage in children and adults in the acute stage of the disease the drug is instilled into the conjunctival sac 1-2 drops 6-8 times a day. As the relief of inflammation number instillation was reduced to 2-3 times a day until the disappearance of symptoms.
The syndrome of “dry” eyes drug is used daily by digging into the diseased eye by 2 drops 1- 2 times daily until 25-30 days until disappearance of symptoms.
For the prevention and treatment of complications after excimer laser corneal refractive surgery the drug is used daily by digging into the eyes of 1-2 drops 2 times a day, starting from the day of operation for 10 days.
For the prevention of graft disease and prevent recurrence of herpetic keratitis after keratoplasty drug is used daily by digging in 1-2 drops in the operated eye 3-4 times a day during the first two weeks after surgery.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | Firn M |










There are no reviews yet.