- No products in the cart.
Nizoral cream 20 mg / g, 15g tube
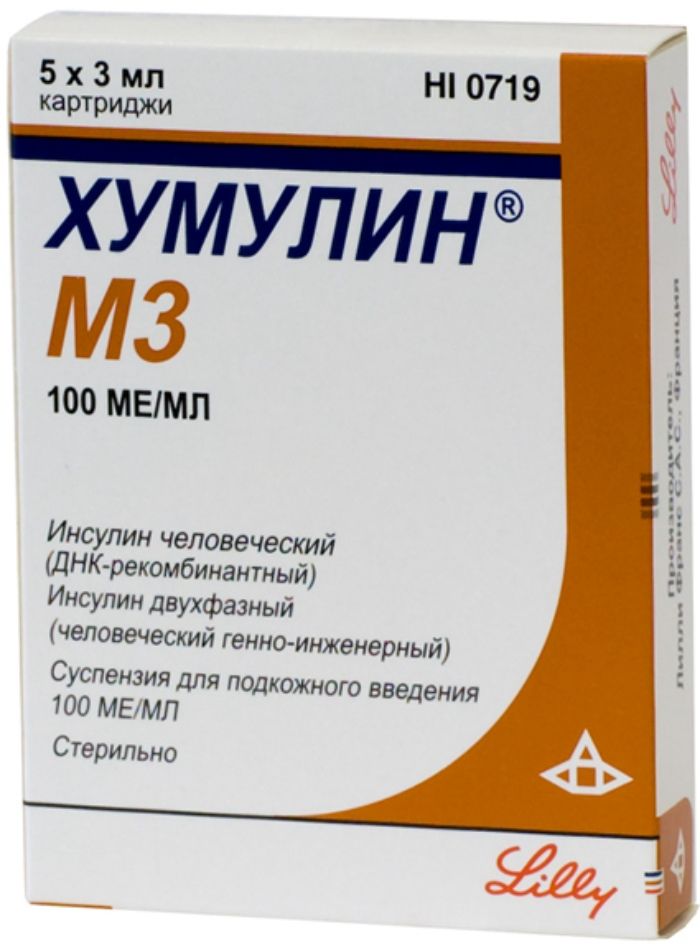
Humulin m3 for suspension and / p / 100me / ml 3ml cartridge 5 pcs
$23.05

Ekzifin gel naruzhn.prim-I 1% 15g tube
$7.89
$12.57
Nizoral cream 20 mg / g, 15g tube
Description
Composition
Active substance:
1 g of cream contains: ketoconazole 20 mg.
Excipients:
Propylene glycol 200 mg (eq. 193 .mu.l), stearyl alcohol 75 mg cetyl alcohol, 20 mg sorbitan stearate 20 mg polysorbate 60 15 mg isopropyl myristate 10 mg sodium sulfite anhydrous 2 mg polysorbate 80 1 mg Purified water to 1 g (637 l).
Description:
White homogeneous cream.
Product form:
Cream for external use 2%.
15 g of cream in an aluminum tube. One tube in a cardboard package, along with instructions for medical use of the drug.
Contraindications
Known hypersensitivity to ketoconazole or any of the auxiliary components of the preparation.
Dosage
20 mg / g
Indications
Dermatofitovye skin infections caused by Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis and Epidermophyton floccosum: smooth skin ringworm, jock itch, athlete’s hands and feet,
Skin candidiasis,
Pityriasis versicolor,
Seborrheic dermatitis due Pityrosporum ovale.
Interaction with other drugs
It has not been studied.
Overdose
The application of excessive amounts of cream can cause the development of erythema, edema and burning sensation, which disappear after cessation of therapy.
In case of accidental ingestion is supportive and symptomatic therapy.
pharmachologic effect
Pharmacological group:
Antifungal agent.
Pharmacodynamics:
Ketoconazole – imidazoldioksolana synthetic derivative having fungicidal or mikostaticheskim activity against dermatophytes such as Trichophyton sp, Epidermophyton floccosum, and Microsporum sp, and against yeast, especially against Malassezia spp…
NIZORAL® cream 2% very quickly affects the itching when dermatophyte and yeast infections, with symptomatic improvement is observed before the first signs of recovery.
Pharmacokinetics:
The concentrations of ketoconazole were not detected in the blood plasma of adult patients after topical application to the skin NIZORAL® cream 2%.
In a study of 19 children with seborrheic dermatitis, wherein about 40 g of a 2% NIZORAL® cream was applied daily on the skin surface area of more than 40%, the concentration of ketoconazole in the plasma of the body surface were determined in 5 children and amounted to from 32 to 133 ng / ml .
Repeated application of the cream in children in large quantities (more than 3 d) there is the likelihood of drug interactions – inhibiting the metabolism of drugs metabolized by CYP3A4 enzyme system, in particular of cisapride are also possible amplification of allergic reactions.
Pregnancy and breast-feeding
Although the application of the cream NIZORALO Ketoconazole does not penetrate through the skin, adequate and well-controlled studies in pregnant women and has not been during the breastfeeding period. Plasma concentrations of ketoconazole were not found after applying the cream on the skin NIZORALO nonpregnant women. There are no known risks associated with the use of cream NIZORALO 2% during pregnancy.
There is no evidence that the drug NIZORALO cream 2% can be dangerous when used in pregnant women or during breast-feeding.
Pregnancy and lactation are used only if the expected benefit to the mother outweighs the potential risk to the fetus and the child, after consultation with the doctor.
Conditions of supply of pharmacies
Without recipe.
side effects
The data below summarizes the information on side effects reported in clinical trials, as well as data on the safety profile of the preparation obtained in the course of its use in clinical practice.
Side effects are grouped according to the classification of organs and organ systems MedDRA (Medical Dictionary for regulatory activities). Criteria for evaluation frequency of side effects: very often (> 1/10), often (> 1/100 and 1/1000 and 1/10000 and
Disorders of immune system: Infrequent – increased sensitivity.
Violations of the skin and subcutaneous tissue disorders: often – burning of the skin; infrequently – bullous rash, contact dermatitis, rash, skin peeling, skin adhesiveness; frequency is unknown – urticaria.
General disorders and application site: often – erythema and itching at the site of application; rarely – bleeding, discomfort, dryness, inflammation, irritation, paraesthesia, application site reactions.
If any of these instructions side effects are compounded, or observed any other side effects not mentioned in the instructions, you should immediately inform your doctor.
special instructions
For external use only.
NIZORAL® cream should not be used in ophthalmic practice.
In order to prevent the cancellation after the termination of long-term treatment with topical corticosteroids syndrome, it is recommended to continue the use of topical corticosteroids in the morning and evening NIZORAL® cream, and then gradually, within 2-3 weeks, abolish steroid therapy.
If the drug has come into disrepair or expiration date – do not throw it in the sewer system or into the street! Place the drug in the bag and place in the trash. These measures will help protect the environment!
The effect on the ability to operate vehicles, machinery
It has not been studied.
Storage conditions
Store at a temperature of from 15 C to 30 C. Keep out of reach of children.
Dosing and Administration
Skin candidiasis, ringworm smooth skin, jock itch, athlete’s foot and toes, pityriasis versicolor: it is recommended to apply NIZORAL® cream once a day to the affected skin and immediately adjacent areas.
Seborrheic dermatitis:
NIZORAL® cream is applied to the affected area once or twice a day depending on the severity of the injury.
Treatment should be continued for a sufficient period of time, at least for a few days after disappearance of all symptoms. The diagnosis should be reconsidered if no clinical improvement is noted after 4 weeks of treatment. Should comply with the general hygiene measures to control sources of infection and reinfection.
The usual duration of treatment is as follows: chromophytosis – 2-3 weeks, yeast infections – 2-3 weeks, jock itch – 2-4 weeks, tinea smooth skin – 3-4 weeks, athlete’s foot – 4-6 weeks.
The usual duration of treatment in the case of seborrheic dermatitis – 2-4 weeks. For maintenance treatment of seborrheic dermatitis cream applied once or twice a week.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | JOHNSON |










There are no reviews yet.