- No products in the cart.
Nasonex spray Nazal. dosed 50mkg / dose vial with 18g 120doz doz.ustr-tion and cap
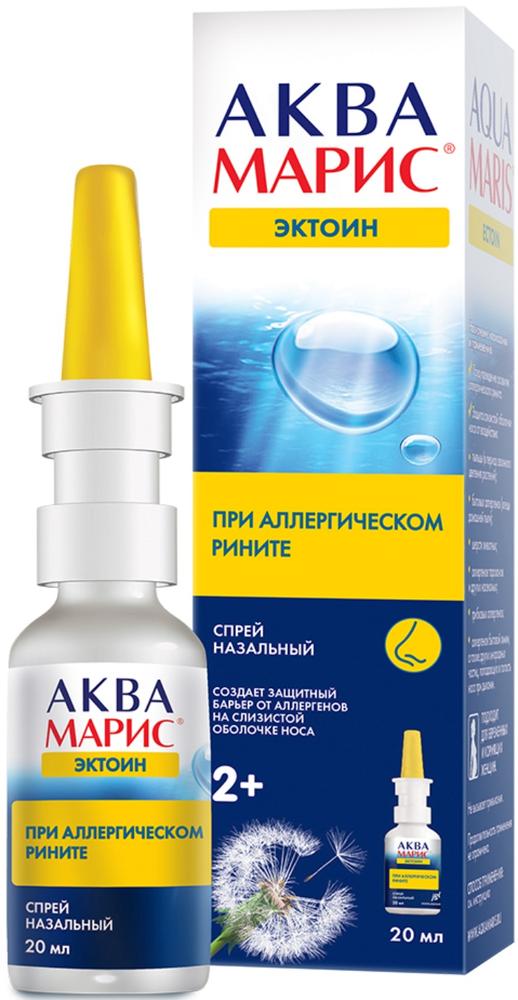
Aqua Maris ectoine nasal spray for the prevention and treatment of allergic rhinitis 20ml
$10.19

Suprastin tab 25mg 20 pc
$2.72
$18.81
Nasonex spray Nazal. dosed 50mkg / dose vial with 18g 120doz doz.ustr-tion and cap
Description
Composition
Active substance:
Mometasone furoate (micronized in the form of monohydrate) 50 mg / dose.
Excipients:
Cellulose dispersion (microcrystalline cellulose treated carmellose sodium), glycerol, citric acid monohydrate, sodium citrate dihydrate, polysorbate 80, benzalkonium chloride (as a 50% solution), phenyl ethanol, purified water.
Description:
SCS for local use. It has anti-inflammatory and anti-allergic effect. The local anti-inflammatory effect of the drug manifested when used at doses at which there is no systemic effects.
Inhibits the release of inflammatory mediators. Increases lipomodulin production, an inhibitor of phospholipase A, which causes the decrease of arachidonic acid release and, accordingly, suppression of the synthesis of the arachidonic acid metabolism products – cyclic endoperoxides, prostaglandins. Prevents the accumulation of neutrophils boundary, which reduces the inflammatory exudation and production of lymphokines inhibits the migration of macrophages, reduces the processes of infiltration and granulation. Reduces inflammation by reducing the formation of chemotactic substance (effect on later reactions allergies), inhibits the development of immediate allergic reaction (due to inhibition of the formation of arachidonic acid metabolites and reducing the release of mast cell mediators of inflammation).
In studies with provocative tests with the application of antigens on nasal mucosa high anti-inflammatory activity of the drug has been demonstrated, both early and late stages of an allergic reaction. When compared with placebo installed reducing histamine and eosinophil activity and a decrease (compared to baseline), number of eosinophils, neutrophils and adhesion proteins of epithelial cells.
Product form:
Vial 120 doses.
Contraindications
– Children under 2 years old; – untreated infection involving a process of the mucosa of the nasal cavity; – newly transferred surgery or trauma of the nose (to wound healing); – pulmonary tuberculosis (including the latent), untreated fungal, bacterial, systemic viral infections (including those caused by Herpes simplex virus with eye disease); – hypersensitivity to the drug.
Dosage
50 ug / dose
Indications
– the treatment of seasonal and perennial allergic rhinitis in adults, adolescents and children from 2 years; – exacerbation of chronic sinusitis in adults (including the elderly) and children from the age of 12 (as an aid in the complex of antibiotic therapy); – prevention of seasonal allergic rhinitis, moderate and severe (recommended for 2-4 weeks before the start of the season dusting).
Interaction with other drugs
Simultaneous application Nasonex loratadine does not alter the concentration of loratadine or its major metabolite in plasma, the plasma is not determined by the presence of mometasone furoate even in minimal concentration.
Nasonex drug interaction studies have not been conducted with other drugs.
pharmachologic effect
Pharmacokinetics:
Intranasal application of systemic bioavailability of the drug is less than 0.1%. Wherein the mometasone furoate is practically determined in blood plasma, even when using highly sensitive methods for determining (a sensitivity threshold of 50 pg / ml). A small quantity of active substance which can get into the gastrointestinal tract by intranasal application, is absorbed to a small extent and actively biotransformed in the “first pass” through the liver.
Conditions of supply of pharmacies
On prescription.
side effects
Side effects noted in the treatment of seasonal and perennial allergic rhinitis: adults – epistaxis (i.e. apparent bleeding, as well as allocating the blood stained mucus or blood clots), pharyngitis, burning sensation in the nose; irritation of nasal mucosa. Nosebleeds usually stop on their own and were not severe; They appeared at a frequency somewhat greater than with placebo (5%), but equal to or smaller than the appointment other investigated SCS for intranasal administration, which were used as active controls (some of them frequency nasalbleedings amounted to 15%) . The incidence of other adverse events was comparable with that for placebo). Children – nosebleeds, headache, feeling of irritation in the nose, sneezing (incidence is comparable with the frequency of adverse events in children on placebo).
Side effects was noted in the application of Nasonex as an adjunct for chronic sinusitis in adults and adolescents: a headache, sore throat, burning sensation in the nose, irritation of the nasal mucosa. Epistaxis was moderately expressed, and the frequency of their occurrence when applied Nasonex was comparable to the frequency nasalbleedings with placebo (5% and 4%, respectively).
special instructions
After Nasonex for 12 months signs of atrophy of the nasal mucosa were observed. When biopsies of nasal mucosa showed that mometasone furoate tended to normalize histology.
In applying the drug for a long time (as with any long-term treatment) requires periodic inspection of the nasal mucosa of upper respiratory physician. With the development of the local bacterial or fungal infection of the nose or pharynx drug treatment is recommended to stop and start specific therapy. Continuing for a long time, stimulation of the nasal mucosa and pharynx is the indication to remove the drug.
With prolonged use of the drug suppressing the symptoms of the hypothalamic-pituitary-adrenal system were observed.
Patients who go to the treatment of nasal spray Nasonex after long-term treatment of corticosteroids systemic effects, require special attention. Cancel systemic corticosteroids in these patients may result in adrenal insufficiency, which may require the adoption of appropriate measures.
During the transition from the treatment of corticosteroids systemic effects to the treatment of nasal spray Nasonex, some patients may experience withdrawal symptoms, use of corticosteroids for systemic use (for example, pain in the joints and / or muscles, fatigue, depression), despite the decrease in the severity of symptoms associated with lesions of the nasal mucosa; such patients should be specially convince the advisability of continuing treatment Nasonex nasal spray. Change of therapy may also identify previously evolved allergic diseases such as allergic conjunctivitis and eczema, previously masked therapy glucocorticoid systemic effects.
Patients undergoing GCS therapy, have a reduced immune reactivity and should be warned of an increased risk of infection when in contact with patients with infectious diseases (including varicella, measles).
Use in Pediatrics
In a placebo-controlled clinical trials in children when Nazoneks applied in a dose of 100 mkg / for a year, growth retardation was observed.
Data on the use of the drug in children under 2 years are not available, so Nasonex can not be recommended for use in this age group.
Storage conditions
At a temperature of 15-25 ° C. (Do not freeze).
Dosing and Administration
For the treatment of seasonal and perennial rhinitis, adult (including senile persons) and children from age 12 designate two injection into each nostril once 1 / (total daily dose – 200 mg). After reaching the desired clinical effect of the drug for maintenance therapy dose of 100 micrograms (1 injection in each nostril once 1 /). If necessary, the dose may be increased to 4 injections in each nostril (total dose – 400 mg). Children aged 2-11 years appoint 50 mg (1 injection) in each nostril once 1 / (total daily dose – 100 mcg).
Positive dynamics of clinical symptoms observed usually during the first 12 hours after the first application of the preparation.
For the treatment of exacerbations of chronic sinusitis in the complex therapy with antibiotics for adults (including elderly people) and children from 12 years appoint 100 mg (2 injections) in each nostril 2 total daily dose – 400 micrograms. If necessary, the daily dose may increase to 800 micrograms (4 injection in each nostril 2). After reducing the symptoms recommended dose reduction.
Stereotype feed drug (in which each pressing is an emission of 100 mg slurry, which corresponds to 50 .mu.g of pure mometasone furoate) is set approximately 6-7 “gauge” taps. If the drug has not been used for 14 days or longer, before use must be re “calibration”.
Prior to use, the vial should be shaken vigorously.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | RX DPA |










There are no reviews yet.