- No products in the cart.
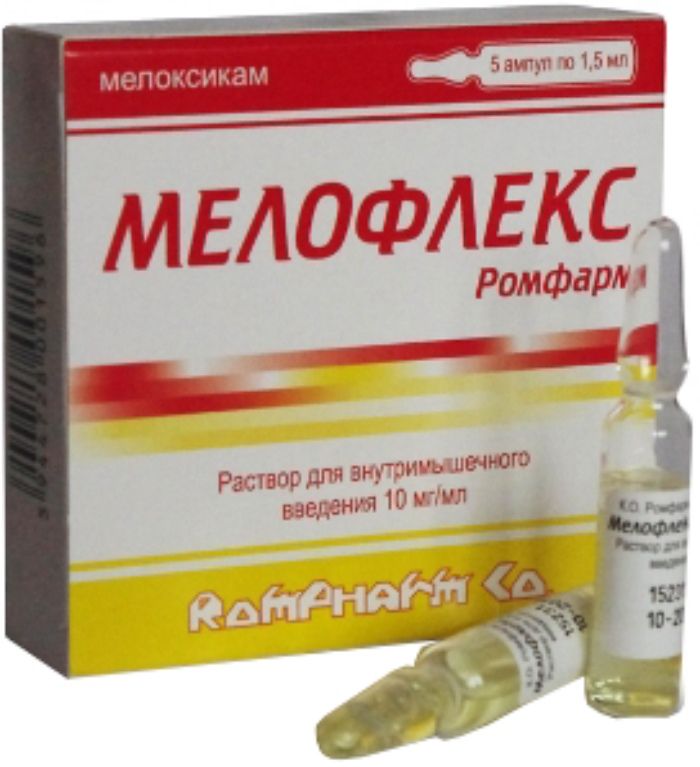
Melofleks Rompharm injection 10mg / ml 1.5ml 5 pcs
$6.73
Melofleks Rompharm injection 10mg / ml 1.5ml 5 pcs
SKU: 2003213202 Categories: Joints and muscles, Medicaments, Pain and inflammation Tags: ampoule, Rompharm
Description
Composition
Active substance:
1 ml contains: meloxicam – 10,000 mg.
Excipients:
Meglumine – 6,250 mg, glikofurfurol – 100,000 mg, poloxamer 188 – 50,000 mg glycine – 5,000 mg Sodium chloride – 3,500 mg, 1 M sodium hydroxide solution – to pH = 8.6 – 9.0, water for injection – up to 1,000 ml.
Description:
Yellow to greenish transparent solution.
Product form:
A solution for intramuscular injection of 10 mg / ml. 1.5 ml of the drug into colorless ampules of 2 ml capacity made of glass of hydrolytic class I with the ring to fracture. On each vial label paste. 3 or 5 ampoules were placed in blisters. 1 contour cellular packaging together with instructions for use placed in a cardboard box.
Contraindications
– hypersensitivity to active substance or auxiliary components; – hypersensitivity to other nonsteroidal anti-inflammatory drugs (NSAIDs), complete or partial combination of asthma, recurrent nasal polyposis mucous membrane and paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including history); – erosive and ulcerative lesions of the stomach and duodenum in the acute stage or recently transferred; – inflammatory bowel disease – Crohn’s disease or ulcerative colitis in the acute stage; – severe hepatic and cardiac failure; – severe renal failure (unless hemodialysis: creatinine clearance less than 30 mL / mission, as well as the confirmed hyperkalemia); – Active liver disease; – active gastrointestinal bleeding, cerebrovascular bleeding recently transferred or established diagnosis of diseases of the blood coagulation system; – children’s age (18 years); – pregnancy; – the period of breast-feeding; – treatment of perioperative pain during coronary artery bypass surgery; – concomitant treatment with anticoagulants (because of the risk of intramuscular hematoma formation).
Precautions – diseases of the gastrointestinal tract (GIT) a history (the presence of N. pulori infection); 6 – Chronic heart failure (CHF); – renal failure (creatinine clearance of 30-60 ml / min); – Coronary heart disease (CHD); – cerebrovascular disease; – dyslipidemia / hyperlipidemia; – diabetes; – concomitant therapy following medications: anticoagulants, oral glucocorticoids, antiplatelet agents, selective serotonin reuptake inhibitor; – peripheral arterial disease; – elderly age; – long-term use of NSAIDs; – smoking; – Frequent use of alcohol.
Dosage
10 mg / ml
Indications
The starting therapy and short-term symptomatic treatment of: – osteoarthritis (arthrosis, degenerative joint disease); – rheumatoid arthritis; – ankylosing spondylitis; – other inflammatory and degenerative diseases of the musculoskeletal system such as arthropathies, dorsopathies (e.g., sciatica, pain in the lower back, shoulder periarthritis) accompanied by pain.
Interaction with other drugs
Other inhibitors of prostaglandin synthesis, including corticosteroids and salicylates – simultaneous with meloxicam increases the risk of formation of ulcers in the gastrointestinal tract and gastrointestinal bleeding (due to synergistic actions). Simultaneous treatment with other NSAIDs is not recommended. Anticoagulants for oral administration for systemic use heparin, thrombolytic agents – simultaneous with meloxicam increases the risk of bleeding. In the case of simultaneous application of careful monitoring of the blood coagulation system. Antiplatelet drugs, selective serotonin reuptake inhibitors – simultaneous with meloxicam increases the risk of bleeding due to inhibition of platelet function. In the case of simultaneous application of careful monitoring of the blood coagulation system. lithium Formulations: NSAIDs increase the concentration of lithium in the blood plasma, by reducing its excretion by the kidneys. The simultaneous use of meloxicam with lithium therapy is not recommended. If necessary, the simultaneous use recommended careful monitoring plasma lithium concentration throughout the course of the application of drugs lithium. Methotrexate: Methotrexate NSAIDs reduce the secretion by the kidneys, thereby increasing its concentration in blood plasma. The simultaneous use of meloxicam and methotrexate (in a dose of 15 mg per week) did not recommended. In the case of simultaneous use of careful monitoring of renal function and blood formula. Meloxicam may enhance methotrexate hematologic toxicity, particularly in patients with impaired renal function. Contraception – there is evidence that NSAIDs may reduce the effectiveness of intrauterine contraceptive devices, but this is not proven. Diuretics – the use of NSAIDs in the case of dehydration of patients with a risk of developing acute renal failure. Antihypertensive drugs (beta-blockers, angiotensin converting enzyme [ACE], vasodilators, diuretics). NSAIDs reduce the effect of antihypertensive agents due to inhibition of prostaglandins having vasodilating properties. Receptor antagonists of angiotensin II, and ACE inhibitors when used in conjunction with NSAIDs enhance reduction of glomerular filtration, which can lead to acute renal failure, especially in patients with impaired renal function. Cholestyramine, linking meloxicam in the gastrointestinal tract, leading to its more rapid removal. Pemetrexed – while the use of meloxicam and pemetrexed in patients with a creatinine clearance of 45 to 79 ml / min should stop reception of meloxicam five days before the beginning of the reception and pemetrexed may resume after 2 days after admission. If there is a need for joint use of meloxicam and pemetrexed, such patients should be carefully monitored, especially in respect of myelosuppression and any side effects from the gastrointestinal tract. In patients with a creatinine clearance less than 45 mL / min reception meloxicam together with pemetrexed is not recommended. NSAIDs, exerting effects on renal prostaglandins may enhance the nephrotoxicity of cyclosporin. When used in conjunction with meloxicam drugs which have a certain ability to inhibit CYP2C9, and / or CYP3A4 (or metabolized by the participation of these enzymes), such as derivatives of sulphonylurea or probenecid, should take into account the possibility of a pharmacokinetic interaction. When combined with anti-diabetic agents for oral administration (e.g., sulfonylureas, nateglinide) possible interactions mediated CYP2C9, which may lead to increased concentrations of both of these drugs, and meloxicam in blood. Patients taking concomitant meloxicam with sulfonylurea or nateglinide should carefully monitor blood sugar levels due to the possibility of hypoglycemia. With simultaneous use of antacids, cimetidine, furosemide and digoxin significant pharmacokinetic interactions have been identified.
Overdose
Data on cases of symptoms associated with overdose of meloxicam, have accumulated enough. Likely to present symptoms characteristic of an overdose of NSAIDs in severe cases, drowsiness, impaired consciousness, nausea, vomiting, epigastric pain, gastrointestinal bleeding, acute renal failure, changes in blood pressure, respiratory arrest, asystole. Antidote treatment is not known. In the case of drug overdose symptomatic therapy should be used. It is known that cholestyramine accelerates the elimination of meloxicam. Forced diuresis, urinary alkalization, ineffective dialysis because of a high degree of binding to plasma proteins meloxicam.
pharmachologic effect
Pharmacological group:
Nonsteroidal anti-inflammatory drugs (NSAIDs).
Pharmacological properties:
Meloxicam is a non-steroidal anti-inflammatory drug, refers to derivatives enolovoy acid and has anti-inflammatory, analgesic and antipyretic effect. Pronounced antiinflammatory action of meloxicam is set on all standard models of inflammation. The mechanism of action of meloxicam is its ability to inhibit prostaglandin synthesis – known inflammatory mediators. In vivo meloxicam inhibits prostaglandin synthesis at the site of inflammation to a greater extent than in the gastric mucosa and kidney. These differences are associated with a selective inhibition of cyclooxygenase-2 (COX-2) over cyclooxygenase-1 (COX-1). It is believed that inhibition of COX-2 provides therapeutic effects of NSAIDs, while the inhibition constant present isoenzyme COX-1 can be a cause of side effects in the stomach and kidney. Meloxicam selectivity for COX-2 was confirmed in various test systems as in vitro, and ex vivo. Selective meloxicam ability to inhibit COX-2 is shown when used as a test system in vitro human whole blood. It has been established that meloxicam (at doses of 7.5 and 15 mg) actively inhibited COX-2 by providing a greater inhibitory effect on prostaglandin E2 production stimulated by lipopolysaccharide (reaction controlled by COX-2) than for the products of a thromboxane involved in blood clotting (reaction controlled by COX-1). These effects depend on the dose. The studies demonstrated ex vivo that meloxicam at recommended doses had no effect on platelet aggregation, and bleeding time. In clinical studies, side effects from the gastrointestinal tract in general occurred less frequently when receiving meloxicam at doses of 7.5 and 15 mg than the reception of other NSAIDs, which were compared. This difference in the incidence of side effects from the gastrointestinal tract is mainly due to the fact that when taking meloxicam rarely observed phenomena such as dyspepsia, vomiting, nausea, abdominal pain. The frequency of the perforations in the upper gastrointestinal tract ulceration and bleeding, are contacted with meloxicam, it was low and was dependent on the dose of the drug.
Pharmacokinetics:
Absorption Meloxicam is completely absorbed following intramuscular administration. Relative bioavailability compared to oral bioavailability is almost 100%. Therefore, when switching from injection to oral dosage forms do not require correction. After administration of 15 mg of the drug intramuscularly maximum plasma concentration is 1.6-1.8 g / ml and is achieved in about 60-90 minutes. Meloxicam distribution very well bound to plasma proteins, particularly albumin (99%). Penetrates into the synovial fluid, synovial fluid concentration is about 50% of plasma concentrations. The volume of distribution (Vd) is low, an average of 11 liters. Interindividual differences account for 7-20%. Metabolism Meloxicam is almost completely metabolized in the liver to form 4 pharmacologically inactive derivatives. The main metabolite, 5`-karboksimeloksikam (60% of the dose), formed by oxidation of an intermediate metabolite, 5`-gidroksimetilmeloksikama which is also excreted, but to a lesser extent (9% of the dose). In vitro studies have shown that this metabolic transformation plays an important role of CYP2C9, additional important isoenzyme CYP3A4. The formation of two other metabolites (components, respectively, 16% and 4% of the dose) participates peroxidase activity which probably varies individually.
Excretion Excreted equally through the intestine and the kidney, mainly in the form of metabolites. In unaltered output from the feces of less than 5% of the daily dose in the urine as unchanged drug is detected only in trace amounts. The average half-life (T1 / 2) of meloxicam is 13-25 hours. Plasma clearance averages 12.7 ml / min after a single application. Meloxicam exhibits linear pharmacokinetics in doses of 7.5-15 mg ingestion or intramuscular administration. Pharmacokinetics in special clinical cases of liver function deficiency and / kidney or liver function deficiency, as well as mild to moderate renal insufficiency significant effect on the pharmacokinetics of meloxicam has not. The rate of excretion from the body of meloxicam significantly higher in patients with moderate renal insufficiency. Meloxicam is less bound to plasma proteins in patients with end-stage renal failure. When ESRD increase in the volume of distribution can result in higher concentrations of free meloxicam, so these patients the daily dose should not exceed 7.5 mg.
Elderly patients Elderly patients compared to younger patients have similar pharmacokinetic parameters. In elderly patients, the mean plasma clearance between the equilibrium state pharmacokinetics is slightly lower than in younger patients. In women older higher values of AUC (area under the curve “concentration-time”) and a long half-life perpiod compared with young patients of both sexes.
Pregnancy and breast-feeding
Use of the drug Melofleks Rompharm contraindicated during pregnancy. Suppression of prostaglandin synthesis may have an adverse effect on pregnancy and fetal development. It is known that NSAIDs penetrate into breast milk, so Melofleks Rompharm contraindicated during breast-feeding. At the time of drug administration must stop breastfeeding. As a drug that inhibits the COX / prostaglandin synthesis, Melofleks Rompharm can affect fertility, and is therefore not recommended for women planning pregnancy. Meloxicam may lead to delay ovulation. Therefore, in women who have difficulty conceiving and passing examination about such problems, it is recommended the abolition of the drug Melofleks Rompharm.
Conditions of supply of pharmacies
Prescription.
side effects
The following describes the side effects, whose connection with the use of the preparation was regarded as possible. Side effects recorded during post-marketing use, whose connection with the drug intake was regarded as possible are marked with “*”. Inside the system-organ classes uses the following categories in the incidence of side effects: very common (> 1/10); common (> 1/100,
special instructions
Patients suffering from diseases of the gastrointestinal tract should be monitored regularly. In the event of ulcerative lesions of the gastrointestinal tract or gastrointestinal bleeding medication should be abolished. Ulcers of the gastrointestinal tract, perforation or bleeding may occur during the use of NSAIDs at any time, as in the presence of warning signs or information about serious gastrointestinal complications in history, and in their absence. The consequences of these complications are generally more serious in the elderly. In applying the drug can develop such serious skin reactions as exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis. Therefore, you should pay special attention to patients reporting of adverse events by the skin and mucous membranes, as well as hypersensitivity reactions to the drug, especially if such reactions were observed during the previous courses of treatment. The development of such reactions occur usually within the first month of treatment. In the case of the first signs of skin rash, mucosal changes or other signs of hypersensitivity should be considered the question of the termination of use of the drug. There are cases when taking NSAIDs increase the risk of serious cardiovascular thrombosis, myocardial infarction, angina, possibly fatal. This risk increases with prolonged use of the drug, as well as in patients with the above diseases in history and predisposed to such diseases. NSAIDs inhibit the renal synthesis of prostaglandins, which are involved in the maintenance of renal perfusion. The use of NSAIDs in patients with reduced renal blood flow or a reduced amount of circulating blood can lead to hidden flowing decompensation of kidney failure. After the cancellation of NSAIDs renal function is usually restored to its original level. The greatest risk for this reaction is subject to elderly patients who have marked dehydration, chronic heart failure, liver cirrhosis, nephrotic syndrome, or acute renal dysfunction, patients simultaneously receiving diuretics, ACE inhibitors, receptor antagonists of angiotensin II, as well as patients, had undergone major surgery, leading to hypovolemia. In these patients at initiation of therapy should be carefully monitored urine output and renal function. The use of NSAIDs in conjunction with diuretics may lead to sodium retention, potassium and water, as well as to reduce the action of natriuretic diuretics. As a result, in predisposed patients may increase symptoms of heart failure or hypertension. Therefore, careful monitoring of the status of these patients, as well as their adequate hydration should be maintained. Prior to the beginning of treatment should be a study of kidney function. В случае проведения комбинированной терапии следует также контролировать функцию почек. При применении лекарственного препарата Мелофлекс Ромфарм (так же как и большинства других НПВП) возможно эпизодическое повышение активности «печеночных» трансаминаз в сыворотке крови или других показателей функции печени. В большинстве случаев это повышение было небольшим и преходящим. Если выявленные изменения существенны или не уменьшаются со временем, препарат следует отменить и проводить наблюдение за выявленными лабораторными изменениями. Ослабленные или истощенные пациенты могут хуже переносить нежелательные явления, в связи с чем, такие пациенты должны тщательно наблюдаться. Подобно другим НПВП, препарат Мелофлекс Ромфарм может маскировать симптомы основного инфекционного заболевания. Как препарат, ингибирующий синтез циклооксигеназы/простагландина, Мелофлекс Ромфарм может оказывать влияние на фертильность, а поэтому не рекомендуется женщинам, имеющим трудности с зачатием. В связи с этим у женщин, проходящих обследование по этому поводу, рекомендуется отмена приема препарата Мелофлекс Ромфарм. У пациентов со слабой или умеренной почечной недостаточностью (клиренс креатинина более 25 мл/мин) коррекции дозы не требуется. У пациентов с циррозом печени (компенсированным) коррекции дозы не требуется.
Effect of the drug for medical use in the ability to operate vehicles, machinery
Специальных клинических исследований влияния мелоксикама на способность управлять автомобилем и механизмами не проводилось. Однако при управлении автомобилем и работе с механизмами следует принимать во внимание возможность развития головокружения, сонливости, нарушения зрения или других нарушений со стороны центральной нервной системы. В период лечения пациентам необходимо соблюдать осторожность при управлении транспортными средствами и занятии другими потенциально опасными видами деятельности, требующими повышенной концентрации внимания и быстроты психомоторных реакций.
Storage conditions
In the dark place at a temperature not higher than 25 C.
Keep out of the reach of children!.
Dosing and Administration
Остеоартрит с болевым синдромом: 7,5 мг в сутки. При необходимости эта доза может быть увеличена до 15 мг в день. Ревматоидный артрит: 15 мг в сутки. В зависимости от лечебного эффекта эта доза может быть снижена до 7,5 мг в день. Анкилозирующий спондилит: 15 мг в сутки. В зависимости от лечебного эффекта эта доза может быть снижена до 7,5 мг в день. У пациентов с повышенным риском побочных реакций (заболевания желудочно-кишечного тракта в анамнезе, наличие факторов риска сердечно- сосудистых заболеваний) рекомендуется начинать лечение с дозы 7,5 мг в день (см. Особые указания). У пациентов с выраженной почечной недостаточностью, находящихся на гемодиализе доза не должна превышать 7,5 мг в день. Общие рекомендации Так как потенциальный риск побочных реакций зависит от дозы и продолжительности лечения следует использовать максимально возможные низкие дозы и длительность применения. Максимальная рекомендуемая суточная доза – 15 мг. Комбинированное применение Не следует применять препарат одновременно с другими НПВП. Суммарная суточная доза мелоксикама, применяемого в виде разных лекарственных форм, не должна превышать 15 мг. Внутримышечное введение препарата показано только в течение первых нескольких дней терапии. В дальнейшем лечение продолжают с применением пероральных лекарственных форм. Рекомендуемая доза составляет 7,5 мг или 15 мг 1 раз в сутки, в зависимости от интенсивности болей и тяжести воспалительного процесса. Препарат вводится посредством глубокой внутримышечной инъекции. Препарат нельзя вводить внутривенно.
Учитывая возможную несовместимость, препарат не следует смешивать в одном шприце с другими лекарственными средствами.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | Rompharm |












There are no reviews yet.