- No products in the cart.
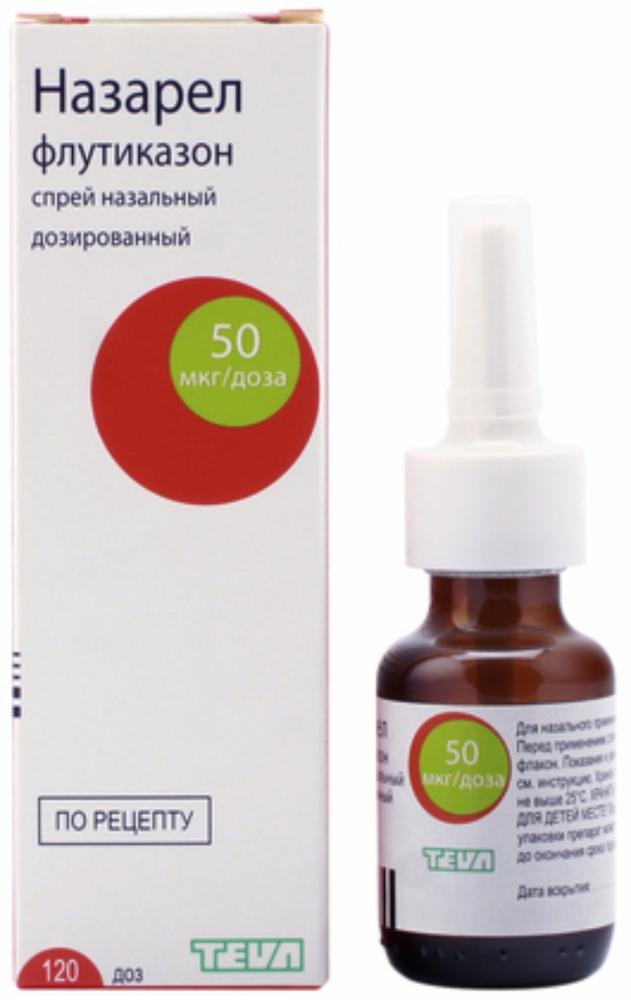
Fliksonaze nasal spray 50mkg / dose vials 60doz
$8.85
Fliksonaze nasal spray 50mkg / dose vials 60doz
Description
Composition
Active substance:
1 dose contains fluticasone propionate (micronized) 50 mg Excipients: Dextrose – 5 mg, Avicel RC591 (microcrystalline cellulose, carmellose sodium) – 1.5 mg, phenylethanol – 0.25 mg Benzalkonium chloride – 0.02 mg Polysorbate 80 – 0,005 mg, hydrochloric acid – until pH 6.3-6.5, purified water – up to 100 mg.
Excipients:
dextrose – 5 mg, Avicel RC591 (microcrystalline cellulose, carmellose sodium) – 1.5 mg, phenylethanol – 0.25 mg Benzalkonium chloride – 0.02 mg Polysorbate 80 – 0,005 mg, hydrochloric acid – to pH 6.3-6.5, purified water – up to 100 mg .
Description:
Nasal spray metered as an opaque white suspension free from foreign particles.
Product form:
60 doses – amber glass vials (1) with a metering device, the adapter and the protective cap – Plastic housings with label-booklet.
Contraindications
Hypersensitivity to fluticasone propionate and other components of the formulation; Children under 4 years of age; recently transferred nasal trauma or surgery and nasal cavity.
Dosage
50 ug / dose
Indications
Treatment of perennial and seasonal allergic rhinitis including hay fever (hay fever) in adults and children from 4 years of pain, feeling of pressure in the sinuses; nasal congestion, sneezing, itchy nose; lacrimation.
Interaction with other drugs
With simultaneous use of fluticasone propionate with ritonavir, which is a potent inhibitor of isozyme CYP3A4, may significantly increase the concentration of fluticasone propionate in plasma. As a result, there is a sharp decrease in the concentration of cortisol in serum. Use of fluticasone propionate inhalation or intranasally and ritonavir results in development of side effects due to systemic effects of corticosteroids, including Cushing’s syndrome and depression of adrenocortical function. Therefore, you should avoid the simultaneous use of fluticasone propionate and ritonavir, except in cases where the potential benefit outweighs the risk of systemic effects. With simultaneous use of fluticasone propionate with other less potent inhibitors of isoenzyme CYP3A4, such as itraconazole and ketoconazole, increases the exposure of fluticasone propionate and increased risk of systemic side effects. We recommend caution and, if possible, avoid long-term joint use of these drugs. Inhibitors of CYP3A4 cause negligible (erythromycin) and minor (ketoconazole) increase the concentration of fluticasone propionate in plasma, which do not entail any significant reduction in serum cortisol concentrations. However, caution should be exercised in the combined use isoenzyme CYP3A4 inhibitors (e.g., ketoconazole) and fluticasone propionate in view of possible increase in the plasma concentration of the latter.
Overdose
Information on acute and chronic overdose are not available. When administered intranasally to healthy volunteers at 2 mg fluticasone propionate 2 times / day for 7 days did not affect the function of the hypothalamic-pituitary-adrenal system (dose 20 times higher therapeutic). Use of the drug in doses higher than those recommended for a long time can lead to temporary suppression of adrenal function. In case of overdose, the patient should seek medical advice.
pharmachologic effect
Pharmacological group:
R01AD08
Pharmacodynamics:
GCS for intranasal use. It has a pronounced anti-inflammatory effect. By nasal administration is not marked systemic action, virtually suppresses the hypothalamic-pituitary-adrenal axis. Significant changes in daily indicator AUC of serum cortisol were found after the administration of fluticasone propionate in a dose of 200 mg / day compared with placebo (ratio: 1.01, 90% CI – confidence interval of 0.9 to 1.14). Fluticasone propionate inflammatory effect is realized as a result of its interaction with receptors glucocorticoids. Inhibits proliferation of mast cells, eosinophils, lymphocytes, macrophages, neutrophils. Fluticasone propionate reduces the production of inflammatory mediators and a number of biologically active substances (including histamine, prostaglandins, leukotrienes, cytokines) during the early and late phase allergic reactions. It provides rapid anti-inflammatory effect on the nasal mucosa. Antiallergic effect occurs already after 2-4 hours after the first application. Reduces sneezing, itchy nose, runny nose, nasal congestion, discomfort in the sinuses and feeling of pressure around the nose and eyes. In addition, it facilitates the ocular symptoms associated with allergic rhinitis. Reduction of symptoms (especially nasal congestion) is maintained for 24 hours after a single spray application of a dose of 200 micrograms. Fluticasone propionate improves the quality of life of patients, including physical and social activity.
Pharmacokinetics:
Absorption After intranasal administration of fluticasone propionate (200 ug / day) Cmax plasma levels at steady state for most patients are not quantitatively determined by (is less than 0.01 ng / ml). Cmax in plasma is 0.017 ng / ml. The direct absorption from the mucosa of the nasal cavity is unlikely due to the low solubility of the drug in water and most of the ingestion of the drug. When taken orally the absolute bioavailability is low (less than 1%) by a combination of incomplete absorption from the gastrointestinal tract and active metabolism in the “first pass” through the liver. Total systemic absorption, thus, extremely low. Distribution at steady state of fluticasone propionate has a large Vd – about 318 liters. Binding to plasma proteins is high – about 91%. Metabolism Fluticasone propionate is output rapidly from the systemic circulation mainly due to the hepatic metabolism to form an inactive carboxylic acid by isoenzyme CYP3A4. The metabolism of ingested fraction fluticasone propionate in the “first pass” through the liver occurs in the same manner. Excretion Excretion fluticasone propionate is linear over a dose range of from 250 to 1000 g and characterized by a high plasma clearance value (1.1 l / min). Cmax plasma decreases by about 98% within 3-4 hours, and only at very low concentrations in plasma observed final T1 / 2 hours 7.8 Renal clearance of fluticasone propionate is negligible (less than 0.2%), and an inactive metabolite -. Carboxylic acid – less 5%. Fluticasone propionate and its metabolites are mostly excreted in bile via the intestine.
Pregnancy and breast-feeding
Before applying Fliksonaze® the drug during pregnancy and lactation should consult a doctor. Pregnant and lactating women Fliksonaze® drug can be prescribed only if the expected benefit to the patient is greater than any possible risk to the fetus or child.
Conditions of supply of pharmacies
Without recipe
side effects
Determining the frequency of side effects: very common (> 1/10), common (> 1/100 and
special instructions
It is indicated only for intranasal application. For adults and children from 12 years: nasal spray Fliksonaze® not use more than 3 months. If necessary, use more than 3 months should consult a doctor. For children from 4 to 12 years: nasal spray Fliksonaze® not use more than 2 months. If necessary, use more than 2 months, consult your doctor. With prolonged use requires regular monitoring of adrenocortical function. There are reports about the manifestation of systemic effects when using nasal corticosteroids, especially in high doses for a long time. These effects are much less likely than when taken orally. Systemic effects when applied nasal corticosteroids may occur, particularly when used in high doses for a prolonged time. The likelihood of these effects is much lower than when taking corticosteroids by mouth, and they may vary in individual patients and between different corticosteroid preparations. Possible systemic effects may include Cushing’s syndrome, features kushingoida, adrenal suppression, growth retardation in children and adolescents, cataract, glaucoma and more rarely a range of psychological or behavioral effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (in especially in children). In children receiving therapy with some intranasal corticosteroids in authorized doses, growth retardation was observed. It is recommended to regularly monitor the growth of children receiving prolonged treatment with intranasal corticosteroids. With growth slowdown should review the treatment to reduce the dose of intranasal corticosteroids, if possible, to the lowest dose for maintaining effective control of symptoms and consult a pediatrician. Concomitant use of ritonavir and fluticasone propionate should be avoided unless the potential benefit to the patient exceeds the potential risk of systemic side effects of corticosteroids. It is recommended to stop taking the drug and consult a doctor if there is no improvement within 4 days. Consultation with a physician is also required if new symptoms appear in a patient, such as severe facial pain, thick nasal discharge, which may indicate an infection and are not associated with allergies. Infections of the nasal cavity or sinuses require appropriate treatment, but are not a contraindication to the use of a nasal spray Fliksonaze®. The majority of patients fluticasone propionate nasal spray eliminates the symptoms of seasonal allergic rhinitis, but in some cases, at very high concentrations in the air of allergens, may need additional therapy. For relief of symptoms with additional therapy may require hand-eye with successful treatment of seasonal allergic rhinitis. In order to achieve maximum therapeutic effect it must adhere to the regular dosage schedule. Care must be taken when transferring patients from systemic corticosteroids therapy for the treatment of fluticasone propionate in the form of a nasal spray, especially if there is a violation of adrenal function on the background of regular monitoring. Fliksonaze®, the aqueous nasal spray contains benzalkonium chloride which may cause bronchospasm. In case of contact with sick chicken pox, measles, and in case of changes from the side view it is advised to stop treatment and seek medical advice. The effect on the ability to drive vehicles and mechanisms in clinical studies received data on the effect of the drug on the ability to drive other mechanisms, however, should consider the adverse effects that can cause drug.
Storage conditions
The drug should be kept out of reach of children at a temperature not higher than 30 ° C.
Dosing and Administration
Fliksonaze® is only intended for intranasal administration. drug should be used regularly to achieve full therapeutic effect. The maximum therapeutic effect appears after 3-4 days of therapy. Adults and children over 12 years 1 week: 2 injections in each nostril of 1 times / day (200 mg / day); from 2 weeks to 3 months: 1 or 2 injections into each nostril 1 time / day (100-200 mg / day). The maximum daily dose – 200 mg / day (no more than 2 injections into each nostril). Elderly patients are administered in the usual dose for an adult. Children aged 4 to 12 years 1 injection into each nostril of 1 times / day (100 mg / day). should not exceed the recommended dose (100 ug / day). For children aged 4 to 12 years, the drug should be used for the shortest period of time necessary to achieve control of symptoms. You should contact your doctor if the child needs to use the drug for a period exceeding 2 months of the year. The maximum daily dose – 100 g (not more than one injection and each nostril). In the absence of effect of the drug in all age groups of patients should consult a doctor. Terms of Use of the drug should be carefully shake the vial before use, take it, placing the index and middle fingers on either side of the tip, and the thumb under the bedplate. For the first use of the drug or a break in its use longer than 1 week check the operation of the nebulizer: forward tip of itself, produce few taps until the tip of the small cloud does not appear. Further it is necessary to clean the nose (vysmorkatsja). Close one nostril and put the tip into the other nostril. Tilt your head slightly forward while holding the vial upright. Then should start to breathe in through the nose and by continuing to inhale, to produce a single touch of a finger to spray the drug. Exhale through the mouth. Repeat this procedure for the second spray in the same nostril, if necessary. Next, repeat the entire procedure described above, insert the tip into the other nostril. After use, the tip should be wet with a clean cloth or handkerchief and close the cap. The sprayer should be washed at least 1 time per week. To do this, carefully remove the tip and rinse it in warm water. Shake off excess water and leave to dry in a warm place. Avoid overheating. Then, carefully set the tip in its place at the top of the vial. Wear protective cap. If the hole is clogged nozzle tip must stay above manner and leave for some time in warm water. Then rinsed under cold running tap water, dried and then put on a bottle. You can not clean the hole with a pin tip or other sharp objects.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | GlaxoSmithKline Helsker |











There are no reviews yet.