- No products in the cart.
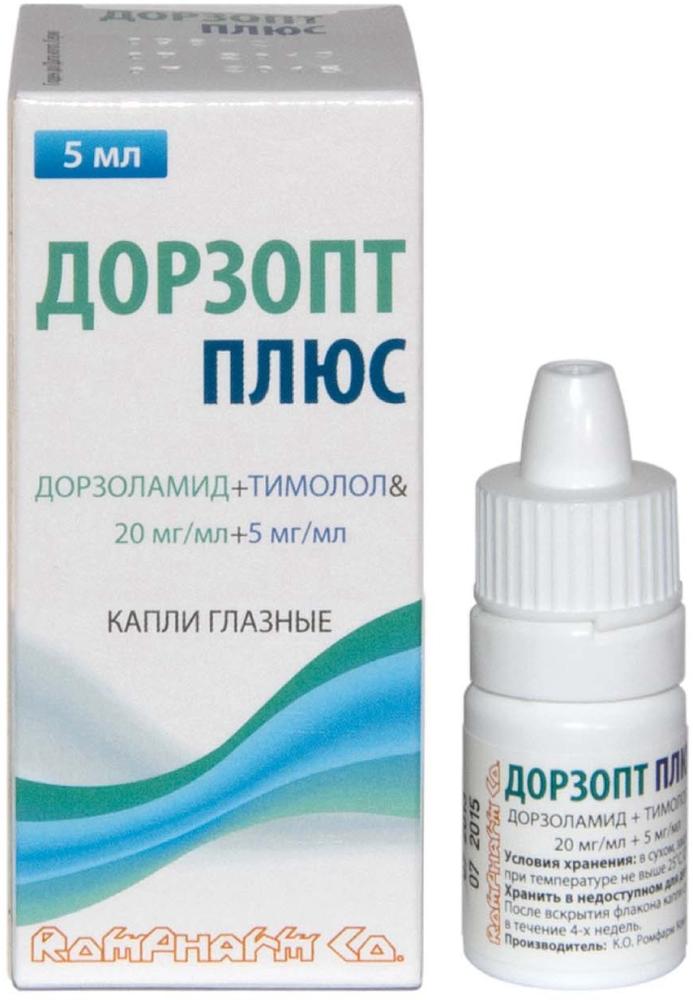
Dorzopt plus drops Ch. 20mg / ml + 5mg / ml-5 ml vial cap.

Zinc sulfate-dia drops Ch. 10ml vial, cap.
$1.84

Katalin tab for prig.kapel Ch. + 0.75mg solvent 15ml vials.
$12.34
$17.05
Dorzopt plus drops Ch. 20mg / ml + 5mg / ml-5 ml vial cap.
SKU: 1917431880 Categories: Glaucoma, Medicaments, Ophthalmology Tags: =+ dorzolamide with timolol, Rompharm
Description
Composition
Active substance:
1 ml contains: dorzolamide hydrochloride (22.26 mg) was converted to 20 mg dorzolamide, timolol maleate (6.84 mg) was converted to 5 mg of timolol ;.
Excipients:
Gietilloza – 1.0 mg citric acid monohydrate – 4.0 mg 1 M sodium hydroxide solution – 0.066 ml mannitol – 20.0 mg Benzalkonium chloride – 0.075 mg, 1 M sodium hydroxide solution / 1 M hydrochloric acid to pH 5.6 + 0.1, purified water – up to 1 ml.
Description:
The clear, colorless or almost colorless, slightly viscous solution.
Product form:
Eye drops of 20 mg / ml + 5 mg / ml. 5 ml of solution in white or transparent polymer-dropper bottle closed with a polymeric cap with a safety ring. In one dropper bottle together with instructions for use in a cardboard package.
Contraindications
Airway hyperresponsiveness, asthma (including history); severe chronic obstructive pulmonary disease (COPD); sinus bradycardia, sinus syndrome, sinoatrial block, atrioventricular block (AV-block) II-III level; severe heart failure; cardiogenic shock; severe renal failure (creatinine clearance less than 30 mL / min), hyperchloremic acidosis; degenerative processes in the cornea; pregnancy, breast-feeding; childhood and adolescence to 18 years (since the efficacy and safety are not well understood); hypersensitivity to any component of the drug.
Carefully
Cardiovascular disease history, including heart failure; I AVblokada degree; COPD mild and moderate severity; severe peripheral circulatory disorders (severe forms of Raynaud’s disease or Raynaud’s syndrome); liver failure; elderly age; diabetes; urolithiasis (including history); hyperthyroidism; disorders of the cornea.
Dosage
20 mg + 5 mg / ml
Indications
Elevated intraocular pressure in open angle glaucoma and pseudoexfoliation glaucoma monotherapy with insufficient efficiency.
Interaction with other drugs
Research Plus Dorzopt drug interactions with other drugs was conducted. However, it is possible to enhance the hypotensive effect and / or development of bradycardia in the combined use of timolol in the form of eye drops and blockers “slow” calcium channels, sympatholytics, beta-blockers, antiarrhythmics (including amiodarone), cardiac glycosides, parasympathomimetics, opioid analgesics and monoamine oxidase inhibitors. When the joint application of timolol and inhibitors isoenzyme CYP2D6 (e.g., quinidine or selective serotonin reuptake inhibitors) reported potentiate the effect of systemic beta blockade (e.g., reduced heart rate, depression). Despite the fact that part of the drug Dorzopt plus carbonic anhydrase inhibitor dorzolamide is used topically, it can penetrate into the systemic circulation. In clinical studies, dorzolamide application as eye drops showed no disorders of acid-base equilibrium. However, when systemic administration of carbonic anhydrase inhibitors, these disorders are known and in some cases they may have an impact on the interaction with other drugs (e.g., amplify toxic reactions when used in high doses of salicylates). Systemic beta-blockers may increase the effects of hypoglycemic agents. Systemic beta-blockers may exacerbate the severity of hypertension, is the effect of clonidine. There are individual data on the development of mydriasis with concomitant use of timolol and epinephrine. There is a possibility amplification known systemic effects of inhibiting carbonic anhydrase in the combined use of local and systemic carbonic anhydrase inhibitors. Since data on the use of such a combination is absent, the combined use of the drug Dorzopt Plus and systemic carbonic anhydrase inhibitors are not recommended.
Overdose
Data on accidental or deliberate overdose of the drug available. There are cases of unintentional overdose of timolol eye drops with the development of systemic symptoms of overdose of beta-blockers when administered systemically: dizziness, headache, shortness of breath, bradycardia, bronchospasm, cardiac arrest. The most expected overdose symptoms of dorzolamide are electrolyte imbalance, development of acidosis, headache, asthenia / fatigue, paresthesia. Treatment: symptomatic and supportive therapy. It should control the concentration of electrolytes (especially potassium) and pH of the blood plasma. Timolol is not displayed during dialysis.
pharmachologic effect
Pharmacological group:
Antiglaucoma agents (carbonic anhydrase inhibitor + beta-adrenergic receptors).
Pharmacodynamics:
Medicament Dorzopt Plus contains two active substances: dorzolamide and timolol, each of which reduces elevated intraocular pressure by reducing the intraocular fluid secretion. The combined effect of these substances in a combined preparation Dorzopt Plus leads to a more pronounced reduction of intraocular pressure. Dorzolamide – selective inhibitor of carbonic anhydrase type II. Inhibition of carbonic anhydrase ciliary body leads to a reduction of intraocular fluid secretion, presumably by reducing the formation of carbonate ions, which in turn leads to a slowing of sodium transport and intraocular fluid. Timolol – nonselective beta-blockers. Although the exact mechanism of action of timolol in reducing intraocular pressure has not yet been installed, a number of studies have shown a preferential reduction in the formation of intraocular fluid, as well as the slight increase its outflow. Reduction of intraocular pressure occurs within 20 min after instillation, reaches a maximum after 2 hours and lasts for at least 24 hours.
Pharmacokinetics:
Dorzolamide Absorption and distribution mainly penetrates into the eye through the cornea (to a lesser extent through the sclera or limb). When applied topically, dorzolamide enters the systemic circulation. With prolonged use dorzolamide accumulates in erythrocytes as a result of selective binding to the carbonic anhydrase II type, maintaining an extremely low concentration of free drug in the plasma. Relationship to plasma proteins – about 33%. Metabolism and excretion of a result of metabolism is transformed into N-dezetilirovanny metabolite is less active against carbonic anhydrase II, but able to block type I carbonic anhydrase. Metabolite also accumulates in erythrocytes, which binds primarily to carbonic anhydrase I type. Excreted by the kidneys in unchanged form and as metabolites. After termination of the drug dorzolamide nonlinear washed erythrocytes from that initially leads to a rapid decrease of its concentration, followed by removal slows. The half-life (T1 / 2) is about 4 months. When you receive inside dorzolamide to simulate maximum systemic exposure at the time of its topical application, the equilibrium state was achieved within 13 weeks. In this case plasma it is practically not found free dorzolamide and its metabolites. Inhibition of carbonic anhydrase of erythrocytes was not sufficient to achieve a pharmacological effect on renal function and respiration. Similar results were observed pharmacological prolonged topical application of dorzolamide. However, some elderly patients with renal failure (creatinine clearance of 30-60 ml / min) showed higher concentration of metabolite in the erythrocytes, but this had no clinical significance.
timolol
The local application of timolol penetrated into the systemic circulation. The concentration of timolol in plasma was studied in 6 patients when applied topically in the form of timolol 0.5% eye drops of 2 times / day. The average maximum concentration (Cmax) after administration in the morning was 0.46 ng / ml after administration day – 0.35 ng / ml.
Pregnancy and breast-feeding
The drug Dorzopt Plus is contraindicated during pregnancy and lactation. Data on the use of dorzolamide dorzolamide during pregnancy is not enough. In studies in rats revealed a teratogenic effect of dorzolamide at doses toxic to pregnant females. It is not known whether dorzolamide passes into breast milk of nursing women. In young females lactating rats receiving dorzolamide, found a decrease in body weight gain. Timolol on the application of the Data timolol during pregnancy is not enough. Epidemiological studies have not revealed the effect of beta-blockers when administered on the formation of congenital malformations, but detected intrauterine growth retardation. In addition, newborns were found signs and symptoms of beta blockade (bradycardia, hypotension, respiratory distress, hypoglycemia) when beta-blockers were applied until delivery. Beta-blockers into breast milk. During the period of breastfeeding is necessary to cancel the use of the drug Dorzopt Plus or stop breastfeeding.
Conditions of supply of pharmacies
Prescription.
side effects
In clinical studies, the drug timolol + dorzolamide is generally well tolerated. Adverse reactions were limited to the already known adverse reactions when using dorzolamide and timolol. Systemic adverse reactions were mild and did not lead to the elimination of the drug. Approximately 1.2% of patients the drug was canceled because of local allergic reactions. Among the most common adverse reactions were local burning or itching sensation in the eye, corneal erosion, conjunctival vascular injection sclera, blurred vision, tearing, distortion of taste. The Post-registration period when using dorzolamide + timolol were observed following undesirable reaction: shortness of breath, respiratory failure, bradycardia, AV-block, choroidal detachment of the eye, nausea, contact dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis. Cases of edema and irreversible destruction of the cornea in patients with chronic corneal defects and / or have undergone intraocular surgery. Known possible adverse reactions of the active ingredients of the preparation:
dorzolamide
From the nervous system: headache, dizziness, asthenia / fatigue, paresthesia.
From a sight organ: inflammation of the eyelid, irritation and flaking century, iridocyclitis, punctuate keratitis, transient myopia (taking place after the drug).
Allergic reactions: angioedema, bronchospasm, urticaria, pruritus, rash. Respiratory system: epistaxis.
Timolol (topical application)
Mental disorders: depression,
Immune system: anaphylaxis, angioedema, urticaria, local or generalized rash.
From the nervous system: tinnitus, paresthesia, headache, asthenia, fatigue, dizziness; insomnia, nightmares, memory loss, increase in symptoms of myasthenia gravis,
The respiratory system: bronchospasm (predominantly in patients with broncho-obstructive pathology prior), cough, chest pain.
On the part of the organ of vision: conjunctivitis, blepharitis, keratitis, decreased corneal sensitivity, a syndrome of “dry eye”; visual disturbances, including refractive changes in the ability of the eye (in some cases due to the cancellation miotikov), diplopia, ptosis.
Cardiac arrhythmias, cardiac arrest.
On the part of the vessels: decrease in blood pressure, fainting, Raynaud’s syndrome, reducing the temperature of the hands and feet.
On the part of the gastrointestinal tract: diarrhea, dyspepsia, dry mouth, throat irritation, abdominal pain.
Skin and subcutaneous tissue disorders: alopecia, psoriasiform rash or exacerbation of psoriasis.
On the part of the musculoskeletal and connective tissue disorders: lameness, systemic lupus erythematosus (SLE).
On the part of genitals and mammary glands: decreased libido, Peyronie’s disease.
General disorders and at the injection site: swelling.
Timolol (systemic application)
From the blood and lymphatic system: netrombotsitopenicheskaya purpura.
From the nervous system: weakness, dizziness, drowsiness, decreased concentration.
From endocrine system: hyperglycemia, hypoglycemia.
The respiratory system: pulmonary edema, wheezing.
Of the heart: lower exercise tolerance, AV-block II-III degree, sinus block, decompensated heart failure, angina progression.
On the part of the vessels: vasodilation.
On the part of the gastrointestinal tract: vomiting.
Skin and subcutaneous tissue disorders: exfoliative dermatitis, itchy skin, increased sweating.
On the part of the kidney and urinary tract: urination disorders.
On the part of the musculoskeletal and connective tissue disorders: pain in the extremities, arthralgia.
On the part of genitals and mammary gland: impotence.
Laboratory and instrumental data: rarely – a slight increase in the concentration of residual nitrogen, potassium, uric acid and triglycerides in blood plasma; a slight decrease in hemoglobin concentration, hematocrit, cholesterol, HDL. These changes are not clinically manifested not progressed.
special instructions
The drug Dorzopt Plus, as well as other ophthalmic drugs used topically, can penetrate into the systemic circulation. As part of the drug is timolol beta-blocker, undesirable reactions during developing in systemic administration of beta-blockers, may occur during topical treatment Dorzopt Plus.
Reactions from the cardiovascular and respiratory system
Before the start of the drug formulation Dorzopt Plus is necessary to ensure adequate control of the cardiovascular system. 8 patients with cardiovascular diseases in history, including heart failure, should be closely monitored for signs of deterioration of these diseases (control heart rate and blood pressure). Account reports of cases of heart failure deaths during treatment with timolol eye drops. When the first signs or symptoms of heart failure, use of the drug should be discontinued Dorzopt Plus. Patients with heart block I degree should be given beta-blockers with caution due to their ability to slow the conduction of impulses. Account reports of cases of bronchospasm with a fatal outcome in patients with bronchial asthma patients during treatment with timolol eye drops. In patients with COPD mild to moderate drug Dorzopt Plus should be used with caution and only if the expected benefit of the treatment outweighs the potential risk. The drug should be used with caution in patients with severe peripheral circulatory disorders (severe disease or Raynaud’s syndrome).
Diabetes
The drug should be used with caution in patients with spontaneous or hypoglycemia in patients with diabetes (especially labile flow) during treatment with insulin or oral hypoglycemic drugs as beta blockers may mask symptoms of hypoglycemia.
hyperthyroidism
Beta-blockers can mask some of the clinical signs of hyperthyroidism (such as tachycardia). If you suspect that the development of hyperthyroidism patients should be monitored carefully. It is necessary to avoid abrupt withdrawal of beta-blockers because of the risk of development of thyrotoxic crisis.
Anesthesia in surgery
The need for the abolition of beta-blockers in the case of the upcoming major surgery has not been proven. The effects of beta-blockers during operation, if necessary, can be eliminated by applying sufficient doses agonists.
Abnormal liver function
Studies of the drug Dorzopt Plus with hepatic insufficiency have not been conducted, and the drug in such patients should be used with caution.
Allergy and hypersensitivity reactions
As with other ophthalmic preparations for topical drug Dorzopt Plus can penetrate into the systemic circulation. Part of the drug is dorzolamide sulfanilamide. Adverse reactions identified in systemic administration of sulfonamides, may occur in the local application of the drug (Stevens-Johnson syndrome and toxic epidermal necrolysis). When you see signs of hypersensitivity reactions, serious the drug should be discontinued. In the treatment of beta-blockers in patients with severe atopy or anaphylactic reactions to various allergens may gain a history response when re-exposed to these allergens. In these patients the use of epinephrine in the standard therapeutic dose for relief of allergic reactions can be inefficient.
concomitant therapy
In applying the drug Dorzopt Plus in patients treated with systemic beta-blockers, it is necessary to consider the possible mutual enhancement of the pharmacological action of drugs in relation to the known systemic effects of beta-blockers, as well as in reducing intraocular pressure. Совместное применение препарата Дорзопт Плюс с другими бета-адреноблокаторами не рекомендуется.
Прекращение терапии
При необходимости отмены местного применения тимолола, как и в случае отмены системных бета-адреноблокаторов, прекращение терапии у пациентов с ишемической болезнью сердца необходимо проводить постепенно.
Нарушения со стороны роговицы
Применяемые в офтальмологии бета-адреноблокаторы могут вызвать сухость слизистой глаза. У пациентов с нарушениями со стороны роговицы препарат должен применяться с осторожностью. У пациентов с низким количеством эндотелиальных клеток имеется повышенный риск развития отека роговицы.
Мочекаменная болезнь
Применение системных ингибиторов карбоангидразы может приводить к нарушению кислотно-щелочного равновесия и сопровождаться уролитиазом, особенно у пациентов с мочекаменной болезнью в анамнезе. В состав препарата Дорзопт Плюс входит ингибитор карбоангидразы, который при местном применении может абсорбироваться и проникать в системный кровоток, поэтому риск развития уролитиаза у пациентов с мочекаменной болезнью в анамнезе при лечении препаратом Дорзопт Плюс может повышаться.
Use in the elderly
В клинических исследованиях разницы эффективности и безопасности дорзоламида+тимолола у пациентов в возрасте более 65 лет по сравнению с более молодыми пациентами не выявлено. Тем не менее, не следует исключать возможности более высокой чувствительности к препарату у некоторых пожилых пациентов.
Использование контактных линз
Препарат Дорзопт Плюс содержит консервант бензалкония хлорид, который может быть причиной возникновения раздражения глаза. Поэтому пациентам перед применением препарата следует удалить мягкие контактные линзы и установить их обратно не ранее, чем через 15 мин после закапывания препарата. Бензалкония хлорид способен обесцвечивать мягкие контактные линзы.
Effect on the ability to drive mechanisms and
В период применения препарата Дорзопт Плюс необходимо воздержаться от управления транспортными средствами и механизмами и занятий потенциально опасными видами деятельности, требующими повышенной концентрации внимания и быстроты психомоторных реакций.
Storage conditions
In a dry place, protected from light at a temperature not higher than 25 C.
In the reach of children !.
Dosing and Administration
Препарат Дорзопт Плюс рекомендуется закапывать по 1 капле в конъюнктивальный мешок глаза (или обоих глаз) 2 раза в сутки. В случае, если препарат Дорзопт Плюс назначается в качестве замены другого офтальмологического препарата для лечения глаукомы, последний необходимо отменить за 1 день до начала терапии препаратом Дорзопт Плюс. В случае совместного применения с другими местными офтальмологическими препаратами, введение препарата Дорзопт Плюс должно проводиться с интервалом в 10 мин. При носослезной окклюзии (закрывании век) на 2 мин после закапывания препарата происходит снижение его системной абсорбции, что может привести к усилению местного действия. Препарат Дорзопт Плюс представляет собой стерильный раствор, поэтому пациенты должны быть проинструктированы, как правильно пользоваться флаконом. Длительность лечения устанавливает врач в зависимости от клинического состояния пациента.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | Rompharm |









There are no reviews yet.