- No products in the cart.
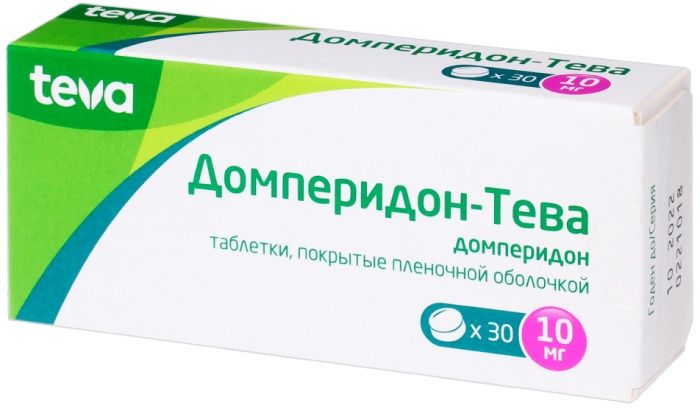
Domperidone-Teva tab p / 10 mg of the film 30 pc
$4.17
Domperidone-Teva tab p / 10 mg of the film 30 pc
SKU: 324429382 Categories: Digestive tract, Medicaments, Motility stimulants Tags: domperidone, TEVA
Description
Composition
Active substance:
domperidone (domperidone maleate) 10.0 (12.73) mg
Excipients:
lactose monohydrate 50.00 mg corn starch 10.00 mg 0.20 mg sodium lauryl sulfate, povidone K30 3.00 mg, 23.32 mg of microcrystalline cellulose, colloidal silicon dioxide 0.25 mg magnesium stearate 0.50 mg ;
shell (hypromellose 2.80 mg 0.30 mg propylene glycol, talc 0.70 mg titanium dioxide E171 1.20 mg).
Description:
White or almost white round biconvex tablets, film-coated, engraved «Do 10 ‘on one side. On the cross-section – the core of white color.
Product form:
Tablets, film-coated, 10 mg.
10 tablets in blisters of PVC / aluminum foil; 3 blisters with instruction for use in a cardboard box.
Contraindications
– Hypersensitivity to domperidone or any other component of the drug.
– prolactin secreting pituitary tumor (prolactinoma).
– Gastrointestinal bleeding.
– Perforation of the gastrointestinal tract.
– Mechanical bowel obstruction.
– Liver failure secondary to severe.
– Patients with cardiac conduction intervals elongation, particularly interval QTc, patients with severe disorders of electrolyte balance, or concomitant heart diseases, e.g., chronic heart failure.
– Concomitant use with drugs that prolong the QT interval, with the exception of apomorphine (see section “Interaction with other medicinal products” and “Cautions”.).
– simultaneous use of ketoconazole for oral administration of erythromycin for oral or other potent inhibitors of isoenzyme CYP3A4, causing the lengthening of the interval QT, such as: fluconazole, clotrimazole, clarithromycin, telithromycin and amiodarone (see “Interaction with other drugs.”).
– Hereditary lactose intolerance, lactase deficiency or glucose-galactose malabsorption.
– The period of breastfeeding.
– Pregnancy.
– Children under 12 years of age or weighing less than 35 kg.
Carefully
Renal failure; simultaneous application of drugs, inducing bradycardia and hypokalemia, and macrolides, prolonging the interval QT – azithromycin and roxithromycin.
Indications
Dyspeptic symptoms, such as nausea, vomiting, feeling of fullness in the epigastric region, discomfort in the upper abdomen and regurgitation of gastric contents.
Interaction with other drugs
The main metabolism of domperidone takes place with the participation of isoenzyme CYP3A4. in vitro study demonstrated that the combined use of drugs, which significantly inhibit the enzyme may cause the appearance of domperidone elevated concentrations in the blood plasma.
Separate studies in vivo pharmacokinetic / pharmacodynamic interactions domperidone with ketoconazole or erythromycin for oral administration in healthy volunteers confirmed the significant inhibition of CYP3A4-mediated metabolism of domperidone data presistemnogo drugs.
Increased risk of QT interval elongation due pharmacodynamic and / or pharmacokinetic interactions
When the joint application of 10 mg of domperidone for oral administration four times daily and ketoconazole 200 mg twice a day, the average prolongation of the interval QT, of 9.8 msec, the individual time points observed during the observation period with the changes in the range of 1,2-17, 5 ms. When the joint application of 10 mg of domperidone for oral administration four times a day, and 500 mg of erythromycin for oral administration three times a day, the mean QT elongation during the observation period was 9.9 ms with changes of individual time points in the range of 1,6-14, 3 ms. Cmax and AUC of domperidone at steady state were increased approximately three-fold in each of these interaction studies. In these studies monotherapy 10 mg domperidone for oral administration four times a day, caused an increase in the average interval QTc, was 1.6 msec (ketoconazole study) and 2.5 ms (research erythromycin), whereas ketoconazole monotherapy (200 mg twice day) monotherapy and erythromycin (500 mg tid) resulted in an increase in the interval QTc, it was 3.8 and 4.9 msec during the observation period, respectively.
Contraindicated for concomitant use with the following drugs
Drugs prolonging the QTc interval:
– antiarrhythmics of class IA (e.g., disopyramide, gidrohinidin, quinidine);
– antiarrhythmic drugs of class III (e.g., amiodarone, dofetilide, dronedarone, ibutilide, sotalol);
– some antipsychotics (e.g., haloperidol, pimozide, sertindole);
– certain antidepressants (e.g., citalopram, escitalopram);
– certain antibiotics (e.g. erythromycin, levofloxacin, moxifloxacin, spiramycin);
– some antifungal drugs (e.g., pentamidine);
– some antimalarials (in particular halofantrine, lumefantrine);
– some gastrointestinal agents (e.g., cisapride, dolasetron, prucalopride);
– Some antihistamines (e.g., mequitazine, mizolastine);
– some of the drugs used in oncology (e.g., toremifene, vandetanib, vincamine);
– some other drugs (e.g., bepridil, difemanil, methadone);
– application in conjunction with apomorphine is only possible if the joint use of an advantage than risks, and only if strictly followed the recommended precautions for joint use of drugs;
– potent inhibitors of CYP3A4 (irrespective of effects prolonging the interval QT), namely HIV protease inhibitors, systemic azole antifungals, some macrolides (e.g., erythromycin, clarithromycin and telithromycin).
Not recommended combined use of the following drugs
Moderate inhibitors of CYP3A4 isoenzyme, namely – diltiazem, verapamil, and some macrolide.
The combined use of these drugs requires caution
Caution should be exercised while the use of drugs induce bradycardia and hypokalemia, and macrolides, prolonging the interval QT: azithromycin and roxithromycin (clarithromycin is contraindicated because it is a potent inhibitor of isozyme CYP3A4).
The combined use with levodopa
Domperidone may be used in conjunction with levodopa, levodopa dose adjustment is required. In a joint application may experience increased levels of levodopa plasma concentrations (up to 30-40%).
The above list of drugs is typical and not exhaustive.
Overdose
Symptoms of overdose include drowsiness, disorientation and extrapyramidal disorders, especially in children.
Treatment. No specific antidote. In the case of overdose can be effective gastric lavage, and the use of activated carbon. It recommended careful monitoring and supportive therapy.
For relief of extrapyramidal disorders may be effective -holinoblokatory m and drugs used to treat parkinsonism.
pharmachologic effect
Pharmacodynamics:
Domperidone – dopamine receptor blocker having antiemetic activity. Antiemetic effect due to a combination of peripheral (gastrokinetic) action and inhibition of dopamine receptors in the brain trigger zone of chemoreceptors located outside the blood-brain barrier in the area postrema. When ingestion of domperidone increases the pressure in the esophagus, antroduodenalnuyu improves motility and accelerates gastric emptying without causing effects on gastric secretion. Domperidone increases prolactin secretion from the pituitary gland.
Pharmacokinetics:
If ingestion fasting domperidone is rapidly absorbed and its maximum plasma concentrations are reached within 30-60 minutes. Low absolute bioavailability after oral administration (about 15%) due to the effect of “first pass” through the liver. While in healthy volunteers increased the bioavailability of domperidone when administered to a meal, patients with complaints on the part of the gastrointestinal tract should take domperidone 15-30 minutes before a meal.
By reducing gastric acidity observed violation of absorption of domperidone. Preliminary receiving cimetidine and sodium bicarbonate reduces the bioavailability of domperidone. If ingestion after eating some more time is required to achieve maximum absorption.
When ingestion of domperidone does not accumulates and does not induce its own metabolism. Domperidone is 91-93% bound to plasma proteins. Domperidone is distributed in various tissues of the body, it is found in breast milk, but does not penetrate the blood-brain barrier. Concentration of domperidone in breast milk is 10-50% of the plasma concentration.
Domperidone metabolism occurs in the liver and in the intestinal wall by hydroxylation and N -dezalkilirovaniya involving isozymes CYP3A4, CYP1A2 and CYP2E1.
Domperidone appears intestine (66%) and kidneys (33%), including: unchanged (10% and 1%, respectively). The half-life is 7-9 hours.
Pharmacokinetics in special patient groups
In patients with serum creatinine concentration of greater than 0.6 mmol / l half-life ranges from 7.4 to 20.8 hours while domperidone plasma concentration decreased.
Pregnancy and breast-feeding
There are limited postmarketing data on the use of domperidone in pregnant women.
Animal study demonstrated reproductive toxicity at the high dose, toxic to the maternal organism. The potential risk for humans is unknown. Therefore, domperidone should be used during pregnancy only if the justification of the intended therapeutic benefit for the mother.
Domperidone is the breast milk from lactating female rats (primarily as metabolites: maximum concentration of 40 and 800 mg / ml after oral or intravenous administration of 2.5 mg / kg, respectively).
Domperidone is excreted in breast milk of women and infants during breastfeeding may receive less than 0.1% of the maternal dose adjusted for body weight. We can not exclude the possibility of adverse effects, particularly cardiac, in infants during the breastfeeding period.
It is necessary to take a decision to stop breastfeeding or withdrawal / abstinence from the drug domperidone, assessing the benefits of breastfeeding for the child and the benefit of therapy for the mother.
Care must be taken in the event of risk factors elongation QTc interval in infants during breastfeeding.
Conditions of supply of pharmacies
Prescription
side effects
Adverse reactions are systematized according to the World Health Organization (WHO): very common (> 1/10), common (by> 1/100 to
special instructions
Preparation Domperidone-Teva contains lactose, so it should not be used in patients with lactose intolerance, galactosemia or impaired absorption of glucose or galactose.
Since only a very small amount of domperidone excreted by the kidneys in an unmodified form, it is unlikely that a correction of a single dose in patients with renal insufficiency. When reapplying dosing frequency to be reduced to 1-2 times per day depending on severity of disease; It may also require dose reduction. During prolonged therapy, such patients should be kept under regular surveillance.
Taking the drug after a meal slows down its absorption.
In applying domperidone possible lengthening QT interval on the electrocardiogram. During post-marketing surveillance for patients receiving domperidone, there were reports of rare cases of QT prolongation and ventricular tachycardia type “pirouette”. These messages contain information about patients with associated risk factors, impaired electrolyte balance and combination therapy, which could contribute to the development of adverse events.
Epidemiological studies have demonstrated that the use of domperidone was associated with an increased risk of serious ventricular arrhythmias or sudden cardiac death (see. Section “Side effects”).
The highest risk was seen in patients older than 60 years, patients treated with daily doses domperidone exceeding 30 mg, and in patients receiving drugs parallel, lengthening QT interval or CYP3A4 inhibitors.
The drug Domperidone-Teva should be used in adults and children at the lowest effective dose.
Preparation Domperidone-Teva is contraindicated for use in patients with lengthening intervals cardiac conduction in the anamnesis, particularly interval QTc, patients with severe disorders of electrolyte balance (hypokalemia, hyperkalemia, hypomagnesaemia) or bradycardia, or patients with underlying heart disease, such as congestive heart failure due to an increased risk of ventricular arrhythmia (see. section “Contraindications”). Electrolyte imbalance (hypokalemia, hyperkalemia, hypomagnesemia) and bradycardia are known as the state, increasing the risk proaritmogennoe.
Domperidone is not suitable for coadministration with drugs that prolong the interval QT, except apomorphine. Use in conjunction with apomorphine only possible if the joint use of the advantage of domperidone apomorphine exceeds the risks and only if strictly followed the recommended precautions for joint use of drugs referred to in the medical application of apomorphine instructions.
Treatment with Teva-Domperidone should be discontinued if there are signs and symptoms that may be associated with cardiac arrhythmias, and patients should consult their physician.
Patients should be advised to immediately report any violations of the heart doctor.
The effect on the ability to operate vehicles, machinery
Before the drug Domperidone-Teva should be careful when driving and busy with other potentially hazardous activities that require high concentration and psychomotor speed reactions due to the possible risk of extrapyramidal disorders, seizures, drowsiness, headache.
Storage conditions
Store at a temperature not higher than 25 ° C.
Keep out of the reach of children.
Dosing and Administration
Inside, before eating, drinking plenty of water. In applying the drug absorption after ingestion of the drug can be slowed down somewhat.
Adults and children over 12 years weighing more than 35 kg 1 tablet 3 times a day. The maximum daily dose of 30 mg / day. The duration of intake should not exceed 1 week. Further the drug is possible after consultation with a doctor.
Patients should try to exercise every dose at the scheduled time.
Omitting the planned dose should be deleted and resume normal dosage regimen of the preparation.
It should not duplicate the reception drug dose to make up for a missed dose.
When liver failure secondary to severe drug application Domperidone-Teva is contraindicated.
When liver failure mild dose adjustment is required.
In renal failure frequency reduction recommended dosing Domperidone-Teva to 1-2 times sutki.Vnutr before eating, drinking plenty of water. In applying the drug absorption after ingestion of the drug can be slowed down somewhat.
Adults and children over 12 years weighing more than 35 kg 1 tablet 3 times a day. The maximum daily dose of 30 mg / day. The duration of intake should not exceed 1 week. Further the drug is possible after consultation with a doctor.
Patients should try to exercise every dose at the scheduled time.
Omitting the planned dose should be deleted and resume normal dosage regimen of the preparation.
It should not duplicate the reception drug dose to make up for a missed dose.
When liver failure secondary to severe drug application Domperidone-Teva is contraindicated.
When liver failure mild dose adjustment is required.
In renal failure frequency reduction recommended dosing Domperidone-Teva of 1-2 times per day.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | TEVA |











There are no reviews yet.