- No products in the cart.
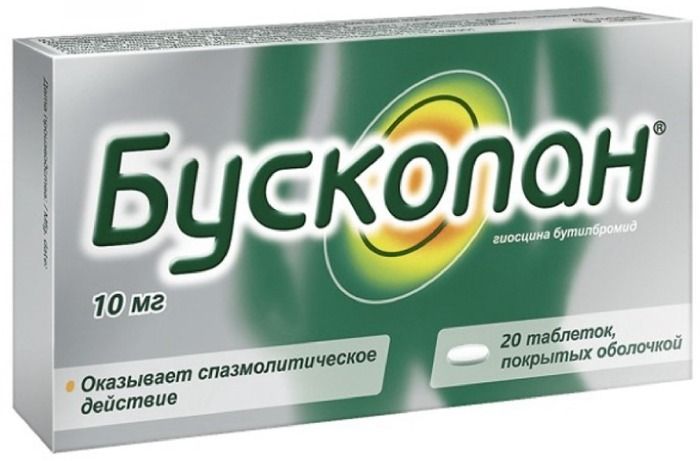
Buscopan tab p / 10 mg of 20 pcs
$10.14
Buscopan tab p / 10 mg of 20 pcs
Description
Composition
Active substance:
1 tablet contains: Hyoscine butylbromide 10 mg.
Excipients:
Calcium hydrogenphosphate anhydrous (calcium hydrogen phosphate) 33 mg; corn starch dried 30 mg; soluble starch (potato starch hydrolyzed) 2 mg; colloidal silicon dioxide 4 mg; tartaric acid 0.5 mg; Stearic acid 0.5 mg.
Sheath: povidone (polyvinylpyrrolidone) 0,505 mg; Sucrose 41.182 mg; 23.671 mg of talc; gum arabic (acacia gum) 2,761 mg; Titanium Dioxide 1,802 mg; Macrogol 6000 (polyethylene glycol) 0,055 mg; Carnauba wax 0.012 mg; white beeswax (white beeswax) 0.012 mg.
Description:
White, round, biconvex tablets, sugar-coated. The smell is almost imperceptible.
Product form:
Film-coated tablets 10 mg
At 10 or 20 tablets in blisters (blister) PVC / AI) -folgi. 1 or 2 blisters with instructions for use in a cardboard box.
Contraindications
Hypersensitivity to hyoscine butylbromide or any other component of the formulation. Myasthenia gravis, megacolon.
Children up to 6 years.
Pregnancy, lactation.
Buscopan tablet contains 41.2 mg of sucrose. The maximum recommended daily dose (10 tablets) contains 411.8 mg of sucrose. Patients with a rare genetic disorders (fructose intolerance), such as glucose-galactose malabsorption or sucrase-isomaltase deficiency should not take the drug.
Carefully.
It should be used with caution in preparation in the following clinical situations: suspected ileus (including pyloric stenosis); urinary tract obstruction (in t. h. benign prostatic hyperplasia), tachyarrhythmias (including atrial tachyarrhythmia), angle-closure glaucoma.
Dosage
10 mg
Indications
Renal colic, biliary colic, spastic dyskinesia of the biliary tract and gallbladder, cholecystitis, intestinal colic, pilorospazm, peptic ulcer and 12 duodenal ulcer in the acute phase (in the complex therapy), tuberculosis.
Interaction with other drugs
Enhances anticholinergic effects of tricyclic antidepressants, antihistamine drugs, quinidine, amantadine, disopyramide, et al. Anticholinergic drugs (including ipratropium bromide, tiotropium bromide). Dopamine antagonists (including metoclopramide) – weakening effect on the digestive tract of both drugs. May exacerbate tachycardia caused by beta-stimulants.
Overdose
Symptoms: dry mouth, urinary retention, skin flushing, tachycardia, inhibition of gastrointestinal motility, transient visual disturbances, paralysis of respiratory muscles.
Treatment: administering cholinomimetics (neostigmine / v or v / m 0.5-2.5 mg), paralysis of the respiratory muscles – intubation, mechanical ventilation, bladder catheterization, symptomatic therapy and poddrezhivayuschaya.
pharmachologic effect
Pharmacological group:
M-holinoblokator.
Pharmacodynamics:
M-cholinergic receptor blocker. Provides local antispasmodic effect on the smooth muscles of internal organs (gastrointestinal tract, biliary tract, urinary tract), reduces the secretion of digestive glands. The local spasmolytic action explained ganglioblokiruyuschimi and m-anticholinergic activity of the drug.
As the quaternary ammonium derivative, hyoscine butylbromide penetrates through the BBB, however anticholinergic effect on the central nervous system is offline.
The drug begins to show spasmolytic effect 15 minutes after administration.
Pharmacokinetics:
Suction.
As the quaternary ammonium derivative thereof, and having a high polarity, hyoscine butylbromide slightly absorbed in the digestive tract. After oral administration the absorption of the drug was 8%. Mean absolute bioavailability is less than 1%. After a single application of hyoscine butylbromide inwardly at doses 20-400 mg mean Cmax in plasma attained about 2 h and ranged from 0.11 to 2.04 ng / ml.
Distribution.
Hyoscine butylbromide due to the high affinity m- and n-holinoretseptorami distributed mainly in muscle cells abdominal and pelvic organs, as well as in the intramural ganglia of the abdominal cavity. The degree of binding to plasma proteins (albumin) – low and is about 4.4%. It is found that the product (at a concentration of 1 mmole) in vitro reacted with choline transport (1.4 nmol) in the epithelial cells of the human placenta.
Metabolism and excretion.
Terminal T1 / 2 after a single oral administration at doses of 100-400 mg ranged from 6.2 to 10.6 hours. Metabolism is mainly by hydrolysis of ester bond. After ingestion of the drug occurs excretion in the feces and urine. After applying the drug inside renal elimination is from 2 to 5%, elimination through the intestine – 90%. Renal excretion of metabolites hyoscine butylbromide is less than 0.1% of the dose. Upon receiving the drug orally in doses of 100-400 mg averages clearance ranging from 881 to 1420 l / min, while the Vd corresponding to the same range of doses ranging from 6.13 to 11.3? 105 l, which may be due to low systemic bioavailability. Metabolites excreted in the urine, weakly associated with m-cholinergic receptors, so they are not active and do not possess pharmacological properties.
Pregnancy and breast-feeding
Data on the use of the drug during pregnancy and the penetration of the drug and its metabolites in breast milk is limited.
Studies on the effect of the drug on fertility have been conducted.
As it is not recommended as a precautionary measure to use the drug during pregnancy and lactation.
Conditions of supply of pharmacies
Without recipe.
side effects
Many of these unwanted effects may be related to the anticholinergic properties of the drug. Anticholinergic side effects are usually mild and disappear on their own.
Immune system:
Anaphylactic shock, anaphylactic reactions, shortness of breath, skin reactions (e.g., hives, rash, erythema, pruritus) and other manifestations of hypersensitivity
Cardio-vascular system:
Tachycardia
From the digestive system:
Dry mouth
Skin and subcutaneous tissue disorders:
Disgidroticheskaya eczema
From the urinary system:
Urinary retention.
special instructions
In cases where the abdominal pain of unknown origin continues or gets worse, or if at the same time marked symptoms such as fever, nausea, vomiting, change in consistency of stool and frequency of bowel movements, abdominal sensitivity, decreased blood pressure, fainting or blood in the stool, you should immediately seek medical advice.
Effects on ability to road management and utilization mechanisms.
Studies on the effect of the drug on the ability to drive vehicles and management mechanisms are not carried out.
Storage conditions
Store at a temperature not higher than 25 C.
Dosing and Administration
Inside.
If your doctor is not indicated differently, the recommended dosing regimen:
Adults and children over 6 years: 1-2 tablets 3 5 paz a day with water.
The drug should not be used on a daily basis for more than 3 days without consulting a doctor.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | SANOFI AVENTIS GROUP |










There are no reviews yet.