- No products in the cart.
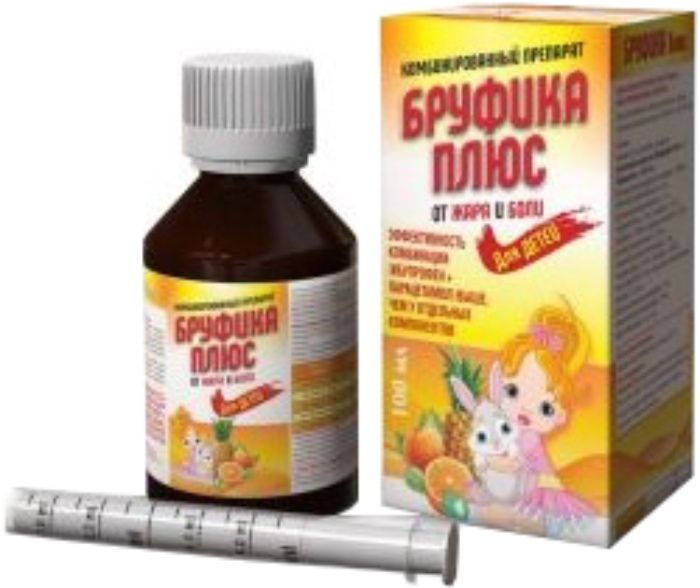
Brufika plus oral suspension for children 100mg / 5ml + 162,5mg / 5ml 100ml
$6.86
Brufika plus oral suspension for children 100mg / 5ml + 162,5mg / 5ml 100ml
Description
Composition
Active substance:
5 ml of suspension contains: ibuprofen – 100 mg paracetamol – 162.5 mg ;.
Excipients:
Sucrose – 3000 mg methyl parahydroxybenzoate – 5 mg propyl parahydroxybenzoate – 1 mg, sodium benzoate – 5 mg Aspartame – 13 mg Sorbitol – 500 mg, carmellose sodium – 6.25 mg, magnesium aluminum silicate – 37.5 mg Citric Acid Monohydrate – 1 mg, sodium citrate – 20 mg glycerol – 100 mg of polysorbate 80 – 5 mg, sunset yellow dye – 0.15 mg orange flavoring – 5 mg, pineapple flavor – 10 mg purified water to 5 mL.
Description:
The viscous suspension light orange or orange color with a characteristic smell.
Product form:
Oral suspension 100 mg + 162.5 mg / 5 ml, 100 ml vials.
Contraindications
The drug should not be used in case of: – hypersensitivity to ibuprofen and / or paracetamol; – erosive and ulcerative diseases of the gastrointestinal tract (including stomach ulcers and 12 duodenal ulcer, Crohn’s disease, ulcerative colitis) or ulcer bleeding in the active phase or in history (two or more confirmed episodes of peptic ulcer or ulcer bleeding) ; – ulcer bleeding or perforation of the gastrointestinal tract in history, triggered by the use of NSAIDs; – full or partial combination of bronchial asthma, recurrent nasal polyposis, and paranasal sinuses, and intolerance to acetylsalicylic acid and other NSAIDs; – coagulation disorders (including hemophilia, hemorrhagic diathesis); – severe renal failure (less than 30 ml / min creatinine clearance); – cerebrovascular or other bleeding; – expressed human liver and kidney; – severe hepatic failure or active liver disease; – decompensated cardiovascular disease; – status after coronary artery bypass surgery; – the confirmed hyperkalemia; – III trimester of pregnancy; – in lactation; – Children under 2 years old.
Carefully:
In the presence of the conditions referred to in this section before using the product Brufika Plus should consult a doctor. – a history of a single episode of gastric ulcer or gastrointestinal bleeding ulcer, gastritis, enteritis, colitis, ulcerative colitis, the presence of infection Helicobacter pylori; – asthma or allergic reactions in the acute stage or history – possibly bronchoconstriction; – severe somatic diseases; systemic lupus erythematosus and other autoimmune connective tissue disease (Sharp’s syndrome) – increased risk of aseptic meningitis; – renal failure, including during dehydration (creatinine clearance of 30-60 ml / min); – fluid retention and edema; – liver dysfunction (including Gilbert’s syndrome); – renal dysfunction; – the genetic absence of the enzyme glucose-6-phosphate dehydrogenase; – liver failure; – arterial hypertension and / or heart failure; – cerebrovascular disease; – dyslipidemia / hyperlipidemia; – diabetes; – peripheral arterial disease; – blood disorders of unknown etiology (leukopenia, anemia, thrombocytopenia); – simultaneous administration of oral corticosteroids (including prednisone), anticoagulants (including warfarin), antiplatelet agents (including acetylsalicylic acid, clopidogrel), selective serotonin reuptake inhibitors (including citalopram, fluoxetine, paroxetine, sertraline); – I-II pregnancy trimester, old age.
Dosage
100 mg + 162.5 mg / 5 ml
Indications
Brufika Plus used in children from 2 years.
As the antipyretic if: acute respiratory diseases; influenza; childhood infectious diseases; postprivivochnyh reactions and other infectious and inflammatory diseases accompanied by fever.
As analgesic mild to moderate intensity with: headache and toothache; migraine; neuralgia; pain in the ears and throat; muscle pain; the pain of injuries, sprains, burns, and other types of pain.
The drug is intended for the symptomatic therapy reduce pain and inflammation at the time of use, does not affect the progression of the disease.
Interaction with other drugs
To avoid the simultaneous application of the following medicaments: – Acetylsalicylic acid: with the exception of low-dose acetylsalicylic acid (not more than 75 mg per day), prescribed by the doctor, since the combined use may increase the risk of side effects. With simultaneous application of reduced anti-inflammatory and antiplatelet effect of acetylsalicylic acid (may increase the incidence of acute coronary insufficiency in patients receiving as, aitiagregantnogo means small dose of acetylsalicylic acid after initiation of the drug).
Precautions used simultaneously with the following medicaments: – anticoagulants and thrombolytic drugs: NSAIDs may enhance the effect of anticoagulants, particularly warfarin and thrombolytic agents. – antihypertensive agents (ACE inhibitors and angiotensin II) and diuretics NSAIDs may reduce efficacy of these groups. Diuretics and ACE inhibitors may increase nefrotoksichnost NSAIDs. – steroids: increased risk of gastrointestinal ulcers and gastrointestinal bleeding. – antiplatelet agents and selective serotonin reuptake inhibitors: increased risk of gastrointestinal bleeding. – cardiac glycosides: co-administration of NSAIDs and cardiac glycosides can lead to aggravation of heart failure, decreased glomerular filtration rate and an increase in concentration of cardiac glycosides in blood plasma. – Lithium drugs: there is evidence of the likelihood of increasing the concentration of lithium in blood plasma during treatment with NSAIDs. – Methotrexate: there is evidence of the likelihood of increasing concentration of methotrexate blood plasma during treatment with NSAIDs. – Cyclosporin: increased risk of nephrotoxicity with concomitant administration of NSAIDs and cyclosporine. – Mifepristone: NSAIDs should be started no earlier than 8-12 days after mifepristone administration as NSAIDs can reduce the efficacy of mifepristone. – Tacrolimus: while the appointment of NSAIDs and tacrolimus may increase the risk of nephrotoxicity. – Zidovudine: the simultaneous use of NSAIDs and zidovudine may increase gematotoksichnosti. There is evidence of increased risk of haemarthrosis and hematoma in HIV-positive patients with hemophilia who received co-treatment with zidovudine and NSAIDs. – quinolone antibiotics series: patients receiving collaborative care NSAIDs and quinolone antibiotics series, may increase the risk of seizures. – barbiturates, carbamazepine, phenytoin, phenytoin, primidone and other anticonvulsants, ethanol, rifampicin, AZT, flumetsinol, phenylbutazone, phenylbutazone, preparations of Hypericum perforatum and other inducers of microsomal oxidation increase the production of hydroxylated active metabolites, causing the possibility of severe liver disease with small overdoses . – inhibitors of microsomal liver enzymes reduce the risk of hepatotoxicity. – under the influence of paracetamol during removal of chloramphenicol (chloramphenicol) increases 5 times, thereby increasing the risk of poisoning chloramphenicol (chloramphenicol). – metoclopramide and domperidone to increase, and cholestyramine reduces the rate of absorption of paracetamol. The drug may reduce the effectiveness of uricosuric drugs.
Overdose
Problems of overdose is very rare, but if you accidentally exceed the recommended dose, then consult a doctor immediately.
Symptoms: abdominal pain, nausea, vomiting, headache, tinnitus, headache and gastrointestinal bleeding, metabolic acidosis, coma, acute renal failure, decreased blood pressure, bradycardia, tachycardia. Perhaps the development of hepatotoxicity with the development gepatonekroz associated with paracetamol.
Treatment: gastric lavage (only one hour after administration), activated carbon, alkaline water, diuresis, administration donators SH-groups, and the synthesis of precursors of glutathione-methionine and N-acetylcysteine. The need for additional therapeutic activities (further administration of methionine, / in administering N-acetyl cysteine) is determined depending on the blood concentration of paracetamol, as well as on the time elapsed after administration. In addition, it shows the symptomatic therapy.
pharmachologic effect
Pharmacological group:
Analgesic combined (NSAIDs are non-narcotic analgesic agent +).
Pharmacological properties:
Analgesic, anti-inflammatory and antipyretic.
Pharmacodynamics:
Combined preparation.
It has analgesic, anti-inflammatory and antipyretic activity. The mechanism of action of ibuprofen and paracetamol due to inhibition of the biosynthesis of prostaglandins – mediators of pain and inflammation.
Effectiveness of the combination (ibuprofen + paracetamol) higher than that of the individual components.
Pharmacokinetics:
Ibuprofen.
Absorption – high. The maximum concentration (TSmax) in plasma reached in 1-2 hours after administration. Relationship to plasma proteins – 90%. The half-life (T1 / 2) -. 2 hours, slowly penetrates the joint cavity, it is retained in the synovial tissue, creating in it a higher concentration than in plasma. After absorption of about 60% pharmacologically inactive R-form is transformed slowly into the active S-shape. Metabolized. Excreted by the kidneys (in unmodified form are not more than 1%) and, to a lesser extent – in the bile.
Paracetamol.
Absorption – high. The maximum concentration (TSmax) in plasma reached after 0.5-2 hours after administration. Relationship to plasma proteins – 15%. It penetrates the blood-brain barrier (BBB).
It is metabolized in the liver (90-95%): 80% unreactive conjugation with glucuronic acid and sulphates to inactive metabolites; 17% undergoes hydroxylation to form 8 active metabolites, which is conjugated with glutathione to form already inactive metabolites. With a lack of glutathione, these metabolites may block the enzyme systems of hepatocytes and cause their death. The metabolism of the drug is also involved isoenzyme CYP2E1. T1 / 2 -. 4.1 h excreted by the kidneys as metabolites, mainly conjugates. In unaltered output of less than 5%. T1 / 2 of 4-5 hours.
Pregnancy and breast-feeding
The drug is intended for use in children is contraindicated in the III trimester of pregnancy. Prior to use in I and II trimester or during breastfeeding should consult a physician.
Conditions of supply of pharmacies
On prescription.
side effects
The risk of side effects can be minimized, if you take a short course of the drug in the lowest effective dose needed to control symptoms.
Qualification frequency of occurrence of adverse reactions performed on the basis of the following criteria: very frequent (> / = 1.10), frequent (from> / = 1/100 to
special instructions
It is recommended to take the drug the maximum possible short-course, and in the lowest effective dose needed to control symptoms.
During long-term treatment is required to monitor patterns of peripheral blood and functional state of the liver and kidneys. When symptoms gastropathy shown careful control, comprising carrying esophagogastroduodenoscopy, total blood (hemoglobin), analysis of fecal occult blood. If necessary, the definition of 17-ketosteroids drug should be discontinued 48 hours prior to the study. During the period of treatment is not recommended intake of ethanol.
Patients with renal failure should consult a physician before using the product, since there is a risk of deterioration of renal function.
Patients with hypertension, including a history of and / or chronic heart failure, it is necessary to consult a physician before using the drug as the drug can cause fluid retention, hypertension and edema.
In the analysis, the determination of uric acid and blood sugar levels, tell your doctor about the use of the drug. Glutathione deficit due to eating disorders, cystic fibrosis, HIV infection, starvation, exhaustion causes the possibility of severe liver injury with small overdoses. The drug should not be used concomitantly with other drugs containing ibuprofen and / or acetaminophen. Patients with rare hereditary fructose intolerance should not take this drug.
INFLUENCE of drugs on driving ability, the mechanism
Patients marking dizziness, drowsiness, confusion or visual disturbances while taking ibuprofen and / or acetaminophen, driving motor vehicles should be avoided or control mechanisms.
Storage conditions
Stored in a dry place at a temperature not higher than 25 C.
Do not freeze!
Keep out of the reach of children.
After opening the bottle of the drug can be stored no more than 6 months.
Dosing and Administration
Brufika Plus is taken orally. The drug is taken at the onset of symptoms (fever or pain).
Before use, carefully shake the vial. For accurate measuring the dose supplied syringe dispensing or measuring spoon.
Dose depends on the child’s age and body weight.
———————————— T ————- ———————- ¬ | body weight (age) | Single dose | + —————- + ——————- —————————— —– + | 10-15 kg (2-3 years) | 5ml | + —————————- ——- + ———————————– + | 16-21 kg ( 4-6 years) | 7.5 mL | + ———————————– + —- ——————————- + | 22-26 kg (7-9 years) | 10 ml | + – ——————————— + —————- ——————- + | 27-32 kg (10-11 years) | 12.5 ml | + ————– ——————— + —————————- ——- + | 33-43 kg (12-14 years) | 15 ml |
L ———————————– + ————- ———————–
The drug is taken three times a day with an interval of 8 hours.
Warning: Do not exceed the specified dose.
Duration of treatment:
– no more than 3 days as an antipyretic;
– not more than 5 days as an analgesic.
If fever or pain persists, consult your doctor.
Methods of application and principle of operation:
USING measuring syringe:
1. Shake well suspension (vial).
2. Insert the syringe into the neck of the bottle.
3. Turn the bottle upside down and gently pull the piston down, picking up the slurry into a syringe to the desired mark.
4. Return the bottle to its original position and remove the syringe, gently turning it.
5. Place the syringe into the child’s mouth and slowly press the plunger, gradually releasing the suspension.
After use, rinse the syringe with warm water and dry it out of reach of children.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | HAYGLANS |











There are no reviews yet.