- No products in the cart.
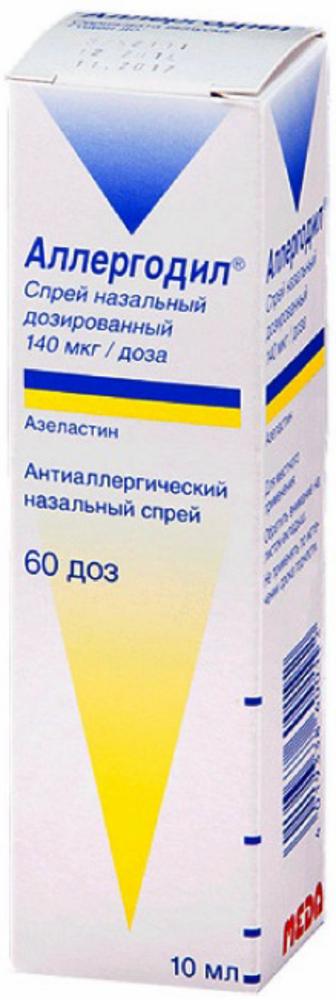
Allergodil spray naz.0.14mg / 10ml dose vials
$11.69
Allergodil spray naz.0.14mg / 10ml dose vials
Description
Composition
Active substance:
1 ml contains: 0.140 mg of azelastine hydrochloride ;.
Excipients:
Hypromellose 0.140 mg; Disodium edetate dihydrate 0.700 mg; 0.061 mg citric acid; Sodium hydrogen phosphate dodecahydrate 0.907 mg; sodium chloride 0.961 mg; Purified water 138.419 mg.
Description:
Transparent, colorless or almost colorless solution.
Product form:
Nasal spray metered 140 ug / dose.
10 ml of a solution in a glass vial with a screw-brown-spray dispenser. 1 vial together with instructions for use placed in a cardboard box.
Contraindications
Hypersensitivity to azelastine and / or other components of the formulation;
In allergic rhinitis and rhinoconjunctivitis – Children up to age 6 years;
When vasomotor rhinitis – Children up to age 12 years.
Dosage
140 micrograms / dose
Indications
The treatment of seasonal and perennial allergic rhinitis (including hayfever) and rhinoconjunctivitis;
The treatment of vasomotor symptoms (year-round non-allergic) rhinitis such as nasal congestion, rhinorrhea, sneezing, postnasal syndrome.
Interaction with other drugs
Intranasal application azelastine not clinically significant interactions with other drugs.
Overdose
At present, cases of overdose by intranasal application is unknown.
pharmachologic effect
Pharmacological group:
Antiallergic agent – H1 histamine receptor blocker.
Pharmacodynamics:
Azelastine, phthalazinone derivative, a long-acting anti-allergic agent. Being selective H1-histamine blockers, has antihistaminic, antiallergic and membrane stabilizing action, reduces capillary permeability and exudation, stabilizes membrane of mast cells and prevents the release of biologically active substances (histamine, serotonin, leukotrienes, platelet activating factor, etc.), Causing bronchospasm and contributing to the development of early and late phase allergic reactions and inflammation. When applied topically negligible systemic exposure. By nasal administration reduces itching and nasal congestion, sneezing and runny nose. Relief of symptoms of allergic rhinitis is noted, starting from 15 min after administration and continues until 12 hours or more.
Clinically significant effects on QT (QTc) interval is not even at long-term use of high doses of azelastine.
Pharmacokinetics:
Bioavailability after intranasal application of about 40%
The maximum concentration (Cmax) in the blood after intranasal application is achieved in 2-3 hours. When applied intranasally in a daily dose of 0.56 mg of azelastine hydrochloride (one injection into each nostril twice a day), the average equilibrium concentration of azelastine hydrochloride in plasma at 2 hours after taking up 0.65 ng / ml.
Doubling da total daily dose of 1.12 mg (two injections in each nostril twice a day) leads to a stable secondary azelastine plasma concentration equal to 1.09 ng / ml.
However, despite the relatively high absorption in patients systemic exposure after intranasal application is about 8 times lower than after oral administration of a daily dose of 4.4 mg of azelastine hydrochloride, which is the oral dose for therapeutic treatment of allergic rhinitis.
Intranasal administration in patients with allergic rhinitis, causes an increase of azelastine plasma levels compared to healthy subjects. Other pharmacokinetic data studied by the oral route.
Communication with blood proteins 80-90%.
It is metabolized in the liver by oxidation with cytochrome P450 to the active metabolite dezmetilazelastanina. Write mainly kidneys as inactive metabolites.
The half-life (T1 / 2) of azelastine – about 20 hours, its active metabolite dezmetilazelastina – about 45 hr.
Pregnancy and breast-feeding
When tested doses, many times higher than the therapeutic range in animals have not produced any evidence of teratogenicity, but because there is no experience of using azelastine in pregnant and breast-feeding is not recommended to use azelastine nasal spray during pregnancy and lactation.
Conditions of supply of pharmacies
Without recipe.
side effects
The incidence of side effects is defined as follows:
Very common:> 1/10;
Often: 1/100;
Uncommon: 1/1000;
Rare: 1/10000;
Very rarely:
Infrequently – weak, passing irritation of the inflamed nasal mucosa, manifested by burning, itching, sneezing, in rare cases, epistaxis.
Very rare – allergic reactions (rash, pruritus, urticaria), weakness, dizziness (may be caused by the disease).
special instructions
In some cases, the use of a nasal spray detected fatigue, of varying severity and weakness, which may be caused and also the basic condition. In these cases, it is not recommended to drive a car and work with dangerous machinery. Drinking alcohol may intensify these effects.
Effects on ability to drive and work with complex mechanisms.
In rare cases, fatigue, tiredness, dizziness or weakness, which may be a consequence of the disease, may develop when using azelastine nasal spray. In these cases, you should avoid driving cars and working with complex mechanisms.
Storage conditions
Store at a temperature of 8-25 ° C.
Keep out of the reach of children.
Dosing and Administration
Intranasally.
Allergic rhinitis and rhinoconjunctivitis.
In the absence of other recommendations for adults and children 6 years and older – on a single oral dose (140 mg / 0.14 ml) in each nostril twice a day, morning and evening. If necessary, adults and children over 12 years: two doses (280 mg / 0.28 ml) in each nostril twice a day, morning and evening. Allergodil applied before symptoms termination and is suitable for prolonged use, but no more than 6 months of continuous treatment.
Vasomotor rhinitis.
Adults and children over 12 years, two doses (280 mg / 0.28 ml) in each nostril twice a day, morning and evening.
Allergodil applied before symptoms termination and is suitable for prolonged use, but no more than 8 weeks of continuous treatment.
Volume of injection (single dose) is 0.14 ml and contains 140 mg of active substance.
Procedure for the application of spray.
1. Remove the protective cap.
2. Before first use, click on the sprayer 2-3 times.
3. Depending on the prescribed dose, inject once or twice in each nostril, holding his head straight.
Information
Appearance may differ from that depicted in the picture. There are contraindications. You need to read the manual or consult with a specialist
Additional information
| Weight | 0.100 kg |
|---|---|
| Manufacturer | HONEY |











There are no reviews yet.